Write a balanced equation for the reaction between \(\text{HNO}_{3}\) and \(\text{KOH}\).
13.2 Acid-base reactions
|
Previous
13.1 Acids and bases
|
Next
13.3 Redox reactions
|
13.2 Acid-base reactions (ESBQY)
The reaction between an acid and a base is known as a neutralisation reaction. Often when an acid and base react a salt and water will be formed. We will look at a few examples of acid-base reactions.
In chemistry the word salt does not mean the white substance that you sprinkle on your food (this white substance is a salt, but not the only salt). A salt (to chemists) is a product of an acid-base reaction and is made up of the cation from the base and the anion from the acid.
-
Hydrochloric acid reacts with sodium hydroxide to form sodium chloride (a salt) and water. Sodium chloride is made up of \(\text{Na}^{+}\) cations from the base \((\text{NaOH})\) and \(\text{Cl}^{-}\) anions from the acid \((\text{HCl})\).
\[\text{HCl (aq)} + \text{NaOH (aq)} \rightarrow \text{H}_{2}\text{O (l)} + \text{NaCl (aq)}\]
-
Hydrogen bromide reacts with potassium hydroxide to form potassium bromide (a salt) and water. Potassium bromide is made up of \(\text{K}^{+}\) cations from the base \((\text{KOH})\) and \(\text{Br}^{-}\) anions from the acid \((\text{HBr})\).
\[\text{HBr (aq)} + \text{KOH (aq)} \rightarrow \text{H}_{2}\text{O (l)} + \text{KBr (aq)}\]
-
Hydrochloric acid reacts with ammonia to form ammonium chloride (a salt). Ammonium chloride is made up of \(\text{NH}_{4}^{+}\) cations from the base \((\text{NH}_{3})\) and \(\text{Cl}^{-}\) anions from the acid \((\text{HCl})\).
\[\text{HCl (aq)} + \text{NH}_{3}\text{(aq)} \rightarrow \text{NH}_{4}\text{Cl (aq)}\]
You should notice that in the first two examples, the base contained \(\text{OH}^{-}\) ions, and therefore the products were a salt and water. \(\text{NaCl}\) (table salt) and \(\text{KBr}\) are both salts. In the third example, \(\text{NH}_{3}\) also acts as a base, despite not having \(\text{OH}^{-}\) ions. A salt is still formed as the only product, but no water is produced.
It is important to realise how useful these neutralisation reactions are. Below are some examples:
-
Domestic uses
Calcium oxide (\(\text{CaO}\)) is a base (all metal oxides are bases) that is put on soil that is too acidic. Powdered limestone \((\text{CaCO}_{3})\) can also be used but its action is much slower and less effective. These substances can also be used on a larger scale in farming and in rivers.
Limestone (white stone or calcium carbonate) is used in pit latrines (or long drops). The limestone is a base that helps to neutralise the acidic waste.
-
Biological uses
Acids in the stomach (e.g. hydrochloric acid) play an important role in helping to digest food. However, when a person has a stomach ulcer, or when there is too much acid in the stomach, these acids can cause a lot of pain. Antacids are taken to neutralise the acids so that they don't burn as much. Antacids are bases which neutralise the acid. Examples of antacids are aluminium hydroxide, magnesium hydroxide (“milk of magnesia”) and sodium bicarbonate (“bicarbonate of soda”). Antacids can also be used to relieve heartburn.
-
Industrial uses
Basic calcium hydroxide (limewater) can be used to absorb harmful acidic \(\text{SO}_{2}\) gas that is released from power stations and from the burning of fossil fuels.
Bee stings are acidic and have a pH between \(\text{5}\) and \(\text{5,5}\). They can be soothed by using substances such as bicarbonate of soda and milk of magnesia. Both bases help to neutralise the acidic bee sting and relieve some of the itchiness!
Acid-base reactions
Aim
To investigate acid-base reactions.
Apparatus and materials
- Volumetric flask
- conical flasks
- sodium hydroxide solution
- hydrochloric acid solution
- pipette
- indicator
Method

-
Use the pipette to add \(\text{20}\) \(\text{ml}\) of the sodium hydroxide solution to a volumetric flask. Fill up to the mark with water and shake well.

-
Measure \(\text{20}\) \(\text{ml}\) of the sodium hydroxide solution into a conical flask. Add a few drops of indicator.
-
Slowly add \(\text{10}\) \(\text{ml}\) of hydrochloric acid. If there is a colour change stop. If not add another \(\text{5}\) \(\text{ml}\). Continue adding \(\text{5}\) \(\text{ml}\) increments until you notice a colour change.
Observations
The solution changes colour after a set amount of hydrochloric acid is added.
In the above experiment you used an indicator to see when the acid had neutralised the base. Indicators are chemical compounds that change colour depending on whether they are in an acid or in a base.
A recommended experiment for informal assessment on discovering natural indicators is included. Learners can test a variety of colourful plants to see what happens to each plant when mixed with an acid or a base. The basic idea is for learners to extract the colour of the plant by boiling the plant matter and then draining the liquid. For substances such as the curry powder, learners can dissolve this in water and for the tea they can brew a cup of tea and then remove the teabag before testing. The resulting liquid can then be tested to see if it is an indicator. An alternative to mixing the acid or base into the liquid is to soak strips of paper in the liquid and then place a drop of the acid or base onto the paper. The experiment below also covers some other substances such as baking powder, vanilla essence and onions. Baking powder fizzes in acids but not in bases. Onions and vanilla essence lose their characteristic smell when in a basic solution.
It is important that learners do not place their faces or noses directly over or into the beaker when smelling the onions and vanilla essence. They must hold the beaker in one hand and use the other hand to waft (i.e. wave their hand back and forth) the smell towards their face.
Acids and bases are corrosive and can cause serious burns so must be handled with care.
Indicators
Aim
To determine which plants and foods can act as indicators.
Apparatus and materials
- Possible indicators: Red cabbage, beetroot, berries (e.g. mulberries), curry powder, red grapes, onions, tea (rooibos or regular), baking powder, vanilla essence
- acids (e.g. vinegar, hydrochloric acid), bases (e.g. ammonia (in many household cleaners)) to test
- Beakers
Method
-
Take a small amount of your first possible indicator (do not use the onions, vanilla essence and baking powder). Boil the substance up until the water has changed colour.
-
Filter the resulting solution into a beaker being careful not to get any plant matter into the beaker. (You can also pour the water through a colander or sieve.)
-
Pour half the resulting coloured solution into a second beaker.
-
Place one beaker onto an A4 sheet of paper labelled “acids”. Place the other beaker onto a sheet of paper labelled “bases”.
-
Repeat with all your other possible indicators (except the onions, vanilla essence and baking powder).
-
Into all the beakers on the acid sheet carefully pour \(\text{5}\) \(\text{ml}\) of acid. Record your observations.
-
Into all the beakers on the base sheet carefully pour \(\text{5}\) \(\text{ml}\) of base. Record your observations.
If you have more than one acid or base then you will need to repeat the above steps to get fresh indicator samples for your second acid or base. Or you can use less of the resulting coloured solution for each acid and base you want to test.
-
Observe the smell of the onions and vanilla essence. Place a small piece of onion into a beaker. This is for testing with the acid. Pour \(\text{5}\) \(\text{ml}\) of acid. Wave your hand over the top of the beaker to blow the air towards your nose. What do you notice about the smell of the onions? Repeat with the vanilla essence.
-
Place a small piece of onion into a beaker. This is for testing with the base. Pour \(\text{5}\) \(\text{ml}\) of base. Wave your hand over the top of the beaker to blow the air towards your nose. What do you notice about the smell of the onions? Repeat with the vanilla essence.
-
Finally place a teaspoon of baking powder into a beaker. Carefully pour \(\text{5}\) \(\text{ml}\) of acid into the beaker. Record your observations. Repeat using the base.
Observations
|
Substance |
Colour |
Results with acid |
Results with base |
|
Red cabbage |
|||
|
Beetroot |
|||
|
Berries |
|||
|
Curry powder |
|||
|
Tea |
|||
|
Red grapes |
|||
|
Onions |
|||
|
Vanilla essence |
|||
|
Baking powder |
You should note that some of the substances change colour in the presence of either an acid or a base. The baking powder fizzes when it is in the acid solution, but no reaction is noted when it is in the base solution. Vanilla essence and onions should lose their characteristic smell when in the base.
Vanilla and onions are known as olfactory (smell) indicators. Olfactory indicators lose their characteristic smell when mixed with acids or bases.
We will now look at three specific types of acid-base reactions. In each of these types of acid-base reaction the acid remains the same but the kind of base changes. We will look at what kind of products are produced when acids react with each of these bases and what the general reaction looks like.
Acid and metal hydroxide (ESBQZ)
When an acid reacts with a metal hydroxide a salt and water are formed. We have already briefly explained this. Some examples are:
- \(\text{HCl (aq)} + \text{NaOH (aq)} \rightarrow \text{H}_{2}\text{O (l)} + \text{NaCl (aq)}\)
- \(2\text{HBr (aq)} + \text{Mg(OH)}_{2} \text{(aq)} \rightarrow 2\text{H}_{2}\text{O (l)} + \text{MgBr}_{2}\text{(aq)}\)
- \(3\text{HCl (aq)} + \text{Al(OH)}_{3}\text{(aq)} \rightarrow 3\text{H}_{2}\text{O (l)} + \text{AlCl}_{3}\text{(aq)}\)
We can write a general equation for this type of reaction: \[n\text{H}^{+}\text{(aq)} + \text{M(OH)}_{n}\text{(aq)} \rightarrow n\text{H}_{2}\text{O (l)} + \text{M}^{n+}\text{(aq)}\] Where \(n\) is the group number of the metal and \(\text{M}\) is the metal.
Acid and metal oxide (ESBR2)
When an acid reacts with a metal oxide a salt and water are also formed. Some examples are:
- \(2\text{HCl (aq)} + \text{Na}_{2}\text{O (aq)} \rightarrow \text{H}_{2}\text{O (l)} + 2\text{NaCl}\)
- \(2\text{HBr (aq)} + \text{MgO} \rightarrow \text{H}_{2}\text{O (l)} + \text{MgBr}_{2}\text{(aq)}\)
- \(6\text{HCl (aq)} + \text{Al}_{2}\text{O}_{3} \text{(aq)} \rightarrow 3\text{H}_{2}\text{O (l)} + 2\text{AlCl}_{3}\text{(aq)}\)
We can write a general equation for the reaction of a metal oxide with an acid: \[2y\text{H}^{+}\text{(aq)} + \text{M}_{x}\text{O}_{y}\text{(aq)} \rightarrow y\text{H}_{2}\text{O (l)} + x\text{M}^{n+}\text{(aq)}\] Where \(n\) is the group number of the metal. The \(x\) and \(y\) represent the ratio in which the metal combines with the oxide and depends on the valency of the metal.
Write a balanced equation for the reaction between \(\text{HBr}\) and \(\text{K}_{2}\text{O}\).
Acid and a metal carbonate (ESBR3)
The reaction of acids with carbonates
Apparatus and materials
- Small amounts of baking powder (sodium bicarbonate)
- hydrochloric acid (dilute) and vinegar
- retort stand
- two test tubes
- one rubber stopper for the test tube
- a delivery tube
- lime water (calcium hydroxide in water)
The experiment should be set up as shown below.
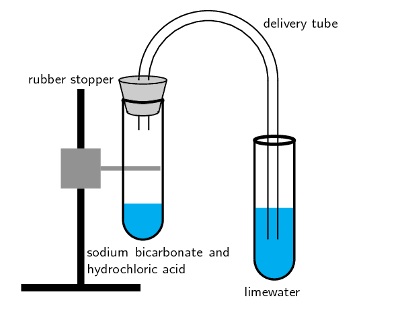
Method
-
Carefully stick the delivery tube through the rubber stopper.
-
Pour limewater into one of the test tubes.
-
Carefully pour a small amount of hydrochloric acid into the other test tube.
-
Add a small amount of sodium carbonate to the acid and seal the test tube with the rubber stopper. Place the other end of the delivery tube into the test tube containing the lime water.
-
Observe what happens to the colour of the limewater.
-
Repeat the above steps, this time using vinegar.
Observations
The clear lime water turns milky meaning that carbon dioxide has been produced. You may not see this for the hydrochloric acid as the reaction may happen to fast.
When an acid reacts with a metal carbonate a salt, carbon dioxide and water are formed. Look at the following examples:
-
Nitric acid reacts with sodium carbonate to form sodium nitrate, carbon dioxide and water.
\[2\text{HNO}_{3}\text{(aq)} + \text{Na}_{2}\text{CO}_{3}\text{(aq)} \rightarrow 2\text{NaNO}_{3}\text{(aq)} + \text{CO}_{2}\text{(g)} + \text{H}_{2}\text{O (l)}\]
-
Sulfuric acid reacts with calcium carbonate to form calcium sulfate, carbon dioxide and water.
\[\text{H}_{2}\text{SO}_{4}\text{(aq)} + \text{CaCO}_{3}\text{(aq)} \rightarrow \text{CaSO}_{4}\text{(s)} + \text{CO}_{2}\text{(g)} + \text{H}_{2}\text{O (l)}\]
-
Hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide and water.
\[2\text{HCl (aq)} + \text{CaCO}_{3}\text{(s)} \rightarrow \text{CaCl}_{2}\text{(aq)} + \text{CO}_{2}\text{(g)} + \text{H}_{2}\text{O (l)}\]
Write a balanced equation for the reaction between \(\text{HCl}\) and \(\text{K}_{2}\text{CO}_{3}\).
Using what we have learnt about acids and bases we can now look at preparing some salts.
Making salts
Aim
To make some salts using acid-base reactions.
Materials
-
hydrochloric acid (\(\text{1}\) \(\text{mol·dm$^{-3}$}\)), sulfuric acid (dilute), sodium hydroxide, copper(II) oxide, calcium carbonate
-
beakers, mass meter, funnels, filter paper, bunsen burner, measuring cylinders
Method
Wear gloves and safety glasses when working with sulfuric acid. Work in a well ventilated room.
Part 1
- Measure out \(\text{20}\) \(\text{ml}\) of hydrochloric acid into a beaker.
- Measure out \(\text{20}\) \(\text{ml}\) of sodium hydroxide and carefully add this to the beaker containing hydrochloric acid.
- Gently heat the resulting solution until all the water has evaporated. You should have a white powder left.
Part 2
- Carefully add \(\text{25}\) \(\text{ml}\) of sulfuric acid to a clean beaker.
- Add about a small amount (about \(\text{0,5}\) \(\text{g}\)) of copper(II) oxide to the beaker containing sulfuric acid. Stir the solution.
- Once all the copper(II) oxide has dissolved, add another small amount of copper(II) oxide. Repeat until no more solid dissolves and there is a small amount of undissolved solid.
- Filter this solution and discard the filter paper.
- Gently heat the resulting liquid. You should get a small amount of solid.
Part 3
- Measure out \(\text{20}\) \(\text{ml}\) of hydrochloric acid into a new beaker.
- Add about a small amount (about \(\text{0,5}\) \(\text{g}\)) of calcium carbonate to the beaker containing hydrochloric acid. Stir the solution.
- Once all the calcium carbonate has dissolved, add another small amount of calcium carbonate. Repeat until no more solid dissolves and there is a small amount of undissolved solid.
- Filter this solution and discard the filter paper.
- Gently heat the resulting liquid. You should get a small amount of solid.
Observations
In the first reaction (hydrochloric acid with sodium hydroxide) the resulting solution was clear. When this solution was heated a small amount of white powder was noted. This powder is sodium chloride.
In the second reaction (sulfuric acid with copper(II) oxide) the resulting solution was blue in colour. When this solution was heated a small amount of white powder was noted. This powder is copper sulfate.
In the third reaction (hydrochloric acid with calcium carbonate) the resulting solution was clear. When this solution was heated a small amount of white powder was noted. This powder is calcium sulfate.
Try write reaction equations for the three reactions above.
Conclusion
We used acid-base reactions to produce different salts.
Acids and bases
For each of the following reactants state what type of acid-base reaction the pair of reactants undergoes and write the balanced reaction equation.
\(\text{HNO}_{3}\) and \(\text{Ca}(\text{OH})_{2}\)
Acid and metal hydroxide
\(2\text{HNO}_{3}\text{(aq)} + \text{Ca(OH)}_{2}\text{(aq)} \rightarrow \text{Ca(NO}_{3}\text{)}_{2}\text{(aq)} + 2\text{H}_{2}\text{O (l)}\)
\(\text{HCl}\) and \(\text{BeO}\)
Acid and metal oxide
\(2\text{HCl (aq)} + \text{BeO (aq)} \rightarrow \text{BeCl}_{2}\text{(aq)} + \text{H}_{2}\text{O (l)}\)
\(\text{HI}\) and \(\text{K}_{2}\text{CO}_{3}\)
Acid and carbonate
\(2\text{HI (aq)} + \text{K}_{2}\text{CO}_{3}\text{(aq)} \rightarrow 2\text{KI (aq)} + \text{H}_{2}\text{O (l)} + \text{CO}_{2}\text{(g)}\)
\(\text{H}_{3}\text{PO}_{4}\) and \(\text{KOH}\)
Acid and metal hydroxide
\(\text{H}_{3}\text{PO}_{4}\text{(aq)} + 3\text{KOH (aq)} \rightarrow \text{K}_{3}\text{PO}_{4}\text{(aq)} + 3\text{H}_{2}\text{O (l)}\)
\(\text{HCl}\) and \(\text{MgCO}_{3}\)
Acid and carbonate
\(2\text{HCl (aq)} + \text{MgCO}_{3}\text{(aq)} \rightarrow \text{MgCl}_{2}\text{(aq)} + \text{H}_{2}\text{O (l)} + \text{CO}_{2}\text{(g)}\)
\(\text{HNO}_{3}\) and \(\text{Al}_{2}\text{O}_{3}\)
Acid and metal oxide
\(6\text{HNO}_{3}\text{(aq)} + \text{Al}_{2}\text{O}_{3}\text{(aq)} \rightarrow 2\text{Al(NO}_{3}\text{)}_{3}\text{(aq)} + 3\text{H}_{2}\text{O (l)}\)
|
Previous
13.1 Acids and bases
|
Table of Contents |
Next
13.3 Redox reactions
|
