What are acids and bases? (ESBQV)
Household acids and bases
Look around your home and school and find examples of acids and bases. Remember that foods can also
be acidic or basic.
Make a list of all the items you find. Why do you think they are acids or bases?
Some common acids and bases, and their chemical formulae, are shown in Table 13.1.
Most acids share certain characteristics, and most bases also share similar characteristics. It is
important to be able to have a definition for acids and bases so that they can be correctly identified
in reactions.
Defining acids and bases (ESBQW)
One of the first things that was noted about acids is that they have a sour taste. Bases were noted to
have a soapy feel and a bitter taste. However you cannot go around tasting and feeling unknown
substances since they may be harmful. Also when chemists started to write down chemical reactions more
practical definitions were needed.
A number of definitions for acids and bases have developed over the years. One of the earliest was the
Arrhenius definition. Arrhenius (1887) noticed that water dissociates (splits up) into
hydronium \((\text{H}_{3}\text{O}^{+})\) and hydroxide \((\text{OH}^{-})\) ions according to the
following equation:
\(2\text{H}_{2}\text{O (l)} \rightarrow \text{H}_{3}\text{O}^{+}\text{(aq)} + \text{OH}^{-}\text{(aq)}\)
For more information on dissociation, refer to Grade 10 (chapter 18: reactions in aqueous
solution).
Arrhenius described an acid as a compound that increases the concentration of
\(\text{H}_{3}\text{O}^{+}\) ions in solution and a base as a compound that increases
the concentration of \(\text{OH}^{-}\) ions in solution.
Look at the following examples showing the dissociation of hydrochloric acid and sodium hydroxide (a
base) respectively:
-
\(\text{HCl (aq)} + \text{H}_{2}\text{O}\text{(l)} \rightarrow
\text{H}_{3}\text{O}^{+}\text{(aq)} + \text{Cl}^{-}\text{(aq)}\)
Hydrochloric acid in water increases the concentration of \(\text{H}_{3}\text{O}^{+}\) ions and
is therefore an acid.
-
\(\text{NaOH (s)} \stackrel{\text{H}_{2}\text{O}}{\longrightarrow} \text{Na}^{+}\text{(aq)} +
\text{OH}^{-}\text{(aq)}\)
Sodium hydroxide in water increases the concentration of \(\text{OH}^{-}\) ions and is therefore
a base.
Note that we write \(\stackrel{\text{H}_{2}\text{O}}{\longrightarrow}\) to indicate that water is
needed for the dissociation.
However, this definition could only be used for acids and bases in water. Since there are many
reactions which do not occur in water it was important to come up with a much broader definition for
acids and bases.
In 1923, Lowry and Bronsted took the work of Arrhenius further to develop a broader definition for acids
and bases. The Bronsted-Lowry model defines acids and bases in terms of their ability
to donate or accept protons.
- Acids
-
A Bronsted-Lowry acid is a substance that gives away protons (hydrogen cations
\(\text{H}^{+}\)), and is therefore called a proton donor.
- Bases
-
A Bronsted-Lowry base is a substance that takes up protons (hydrogen cations
\(\text{H}^{+}\)), and is therefore called a proton acceptor.
Below are some examples:
-
\(\text{HCl (aq)} + \text{NH}_{3}\text{(aq)} \rightarrow \text{NH}_{4}^{+}\text{(aq)} +
\text{Cl}^{-}\text{(aq)}\)
We highlight the chlorine and the nitrogen so that we can follow what happens to these two
elements as they react. We do not highlight the hydrogen atoms as we are interested in how these
change. This colour coding is simply to help you identify the parts of the reaction and does not
represent any specific property of these elements.
\(\text{H}{\color{red}{\text{Cl}}} \text{ (aq)} + {\color{blue}{\text{N}}}\text{H}_{3}\text{(aq)}
\rightarrow {\color{blue}{\text{N}}}\text{H}_{4}^{+}\text{(aq)} +
{\color{red}{\text{Cl}}}^{-}\text{(aq)}\)
In order to decide which substance is a proton donor and which is a proton acceptor, we need to
look at what happens to each reactant. The reaction can be broken down as follows:
\(\text{H}{\color{red}{\text{Cl}}}\text{ (aq)} \rightarrow
{\color{red}{\text{Cl}}}^{-}\text{(aq)}\) and
\({\color{blue}{\text{N}}}\text{H}_{3}\text{(aq)} \rightarrow
{\color{blue}{\text{N}}}\text{H}_{4}^{+}\text{(aq)}\)
From these reactions, it is clear that \(\text{HCl}\) is a proton donor and is therefore
an acid, and that \(\text{NH}_{3}\) is a proton acceptor and is
therefore a base.
-
\(\text{CH}_{3}\text{COOH (aq)} + \text{H}_{2}\text{O (l)} \rightarrow
\text{H}_{3}\text{O}^{+}\text{(aq)} + \text{CH}_{3}\text{COO}^{-}\text{(aq)}\)
Again we highlight the parts of the reactants that we want to follow in this reaction:
\({\color{red}{\text{CH}_{3}\text{COO}}}\text{H (aq)} +
\text{H}_{2}{\color{blue}{\text{O}}}\text{ (l)} \rightarrow
\text{H}_{3}{\color{blue}{\text{O}}}^{+}\text{(aq)} +
{\color{red}{\text{CH}_{3}\text{COO}}}^{-}\text{(aq)}\)
The reaction can be broken down as follows:
\({\color{red}{\text{CH}_{3}\text{COO}}}\text{H (aq)} \rightarrow
{\color{red}{\text{CH}_{3}\text{COO}}}^{-}\text{(aq)}\) and
\(\text{H}_{2}{\color{blue}{\text{O}}}\text{ (l)} \rightarrow
\text{H}_{3}{\color{blue}{\text{O}}}^{+}\text{(aq)}\)
In this reaction, \(\text{CH}_{3}\text{COOH}\) (acetic acid or vinegar) is a proton donor and is
therefore the acid. In this case, water acts as a base because
it accepts a proton to form \(\text{H}_{3}\text{O}^{+}\).
-
\(\text{NH}_{3}\text{(aq)} + \text{H}_{2}\text{O (l)} \rightarrow \text{NH}_{4}^{+}\text{(aq)} +
\text{OH}^{-}\text{(aq)}\)
Again we highlight the parts of the reactants that we want to follow in this reaction:
\({\color{blue}{\text{N}}}\text{H}_{3}\text{(aq)} + \text{H}_{2}{\color{red}{\text{O}}}\text{
(l)} \rightarrow {\color{blue}{\text{N}}}\text{H}_{4}^{+}\text{(aq)} +
{\color{red}{\text{O}}}\text{H}^{-}\text{(aq)}\)
The reaction can be broken down as follows:
\(\text{H}_{2}{\color{red}{\text{O}}}\text{ (l)} \rightarrow
{\color{red}{\text{O}}}\text{H}^{-}\text{(aq)}\) and
\({\color{blue}{\text{N}}}\text{H}_{3}\text{(aq)} \rightarrow
{\color{blue}{\text{N}}}\text{H}_{4}^{+}\text{(aq)}\)
Water donates a proton and is therefore an acid in this reaction. Ammonia
accepts the proton and is therefore the base.
Notice in these examples how we looked at the common elements to break the reaction into two parts. So in
the first example we followed what happened to chlorine to see if it was part of the acid or the base.
And we also followed nitrogen to see if it was part of the acid or the base. You should also notice how
in the reaction for the acid there is one less hydrogen on the right hand side and in the reaction for
the base there is an extra hydrogen on the right hand side.
Amphoteric substances
In examples \(\text{2}\) and \(\text{3}\) above we notice an interesting thing about water. In
example \(\text{2}\) we find that water acts as a base (it accepts a proton). In example
\(\text{3}\) however we see that water acts as an acid (it donates a proton)!
Depending on what water is reacting with it can either react as a base or as an acid. Water is said
to be amphoteric. Water is not unique in this respect, several other substances are
also amphoteric.
- Amphoteric
-
An amphoteric substance is one that can react as either an acid or base.
When we look just at Bronsted-Lowry acids and bases we can also talk about amphiprotic substances
which are a special type of amphoteric substances.
- Amphiprotic
-
An amphiprotic substance is one that can react as either a proton donor (Bronsted-Lowry acid)
or as a proton acceptor (Bronsted-Lowry base). Examples of amphiprotic substances include
water, hydrogen carbonate ion (\(\text{HCO}_{3}^{-}\)) and hydrogen sulfate ion
(\(\text{HSO}_{4}^{-}\)).
Note: You may also see the term ampholyte used to mean a substance
that can act as both an acid and a base. This term is no longer in general use in chemistry.
Polyprotic acids [NOT IN CAPS]
A polyprotic (many protons) acid is an acid that has more than one proton that it can donate. For
example sulfuric acid can donate one proton to form the hydrogen sulfate ion:
\[\text{H}_{2}\text{SO}_{4}\text{(aq)} + \text{OH}^{-}\text{(aq)} \rightarrow
\text{HSO}_{4}^{-}\text{(aq)} + \text{H}_{2}\text{O (l)}\]
Or it can donate two protons to form the sulfate ion:
\[\text{H}_{2}\text{SO}_{4}\text{(aq)} + 2\text{OH}^{-}\text{(aq)} \rightarrow
\text{SO}_{4}^{2-}\text{(aq)} + 2\text{H}_{2}\text{O (l)}\]
In this chapter we will mostly consider monoprotic acids (acids with only one proton to donate). If
you do see a polyprotic acid in a reaction then write the resulting reaction equation with the acid
donating all its protons.
Some examples of polyprotic acids are: \(\text{H}_{2}\text{SO}_{4}\), \(\text{H}_{2}\text{SO}_{3}\),
\(\text{H}_{2}\text{CO}_{3}\) and \(\text{H}_{3}\text{PO}_{4}\).
Acids and bases
Textbook Exercise 13.1
\(\text{HNO}_{3}\text{(aq)} + \text{NH}_{3}\text{(aq)} \rightarrow
\text{NO}_{3}^{-}\text{ (aq)} + \text{NH}_{4}^{+} \text{ (aq)}\)
We break the reaction into two parts:
\(\text{HNO}_{3}\text{ (aq)} \rightarrow \text{NO}_{3}^{-}\text{(aq)}\) and
\(\text{NH}_{3}\text{(aq)} \rightarrow \text{NH}_{4}^{+}\text{(aq)}\)
From this we see that the Bronsted-Lowry acid is \(\text{HNO}_{3}\) and the
Bronsted-Lowry base is \(\text{NH}_{3}\).
\(\text{HBr (aq)} + \text{KOH (aq)} \rightarrow \text{KBr (aq)} + \text{H}_{2}\text{O
(l)}\)
We break the reaction into two parts:
\(\text{HBr (aq)} \rightarrow \text{KBr (aq)}\) and
\(\text{KOH (aq)} \rightarrow \text{H}_{2}\text{O (l)}\)
From this we see that the Bronsted-Lowry acid is \(\text{HBr}\) and the
Bronsted-Lowry base is \(\text{KOH}\).
Write a reaction equation to show \(\text{HCO}_{3}^{-}\) acting as an acid.
\(\text{HCO}_{3}^{-}\text{ (aq)} \rightarrow \text{CO}_{3}^{2-}\text{(aq)} +
\text{H}^{+}\text{(aq)}\)
Write a reaction equation to show \(\text{HCO}_{3}^{-}\) acting as an base.
\(\text{HCO}_{3}^{-}\text{(aq)} + \text{H}^{+}\text{(aq)} \rightarrow
\text{H}_{2}\text{CO}_{3}\text{(aq)}\)
Compounds such as \(\text{HCO}_{3}^{-}\) are \(\ldots\)
Amphoteric
Conjugate acid-base pairs (ESBQX)
Look at the reaction between hydrochloric acid and ammonia to form ammonium and chloride ions (again we
have highlighted the different parts of the equation):
\({\color{red}{\text{HCl}}}\text{ (aq)} + {\color{blue}{\text{NH}_{3}}}\text{(aq)} \rightarrow
{\color{blue}{\text{NH}_{4}^{+}}}\text{(aq)} + {\color{red}{\text{Cl}^{-}}}\text{(aq)}\)
We look at what happens to each of the reactants in the reaction:
\(\text{HCl (aq)} \rightarrow \text{Cl}^{-}\text{(aq)}\) and
\(\text{NH}_{3}\text{(aq)} \rightarrow \text{NH}_{4}^{+}\text{(aq)}\)
We see that \(\text{HCl}\) acts as the acid and \(\text{NH}_{3}\) acts as the base.
But what if we actually had the following reaction:
\({\color{blue}{\text{NH}_{4}^{+}}}\text{(aq)} + {\color{red}{\text{Cl}^{-}}}\text{(aq)} \rightarrow
{\color{red}{\text{HCl}}}\text{ (aq)} + {\color{blue}{\text{NH}_{3}}}\text{(aq)}\)
This is the same reaction as the first one, but the products are now the reactants.
Now if we look at the what happens to each of the reactants we see the following:
\(\text{NH}_{4}^{+}\text{(aq)} \rightarrow \text{NH}_{3}\text{(aq)}\) and
\(\text{Cl}^{-}\text{(aq)} \rightarrow \text{HCl (aq)}\)
We see that \(\text{NH}_{4}^{+}\) acts as the acid and \(\text{Cl}^{-}\) acts as the base.
Up to now you have looked at reactions as starting with the reactants and going to the products. For
acids and bases we also need to consider what happens if we swop the reactants and the products
around. This will help you understand conjugate acid-base pairs.
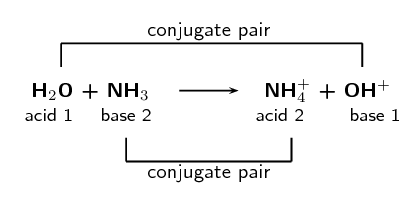
When \(\text{HCl}\) (the acid) loses a proton it forms \(\text{Cl}^{-}\) (the base). And that when
\(\text{Cl}^{-}\) (the base) gains a proton it forms \(\text{HCl}\) (the acid). We call these two
species a conjugate acid-base pair. Similarly \(\text{NH}_{3}\) and
\(\text{NH}_{4}^{+}\) form a conjugate acid-base pair.
The word conjugate means coupled or connected.
We can represent this as:
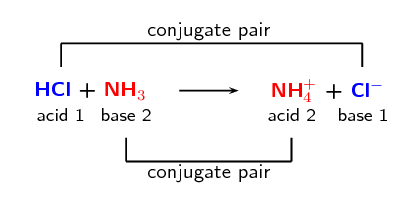
Conjugate acid-base pairs
Using the common acids and bases in Table 13.1,
pick an acid and a base from the list. Write a chemical equation for the reaction of these two
compounds.
Now identify the conjugate acid-base pairs in your chosen reaction. Compare your results to those of
your classmates.
Acids and bases
Textbook Exercise 13.2
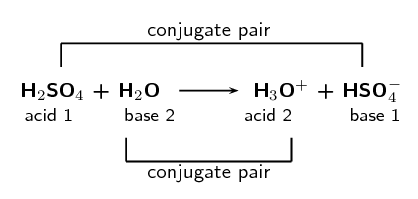
\(\text{H}_{2}\text{SO}_{4}\text{(aq)} + \text{H}_{2}\text{O (l)} \rightarrow
\text{H}_{3}\text{O}^{+}\text{(aq)} + \text{HSO}_{4}^{-}\text{(aq)}\)
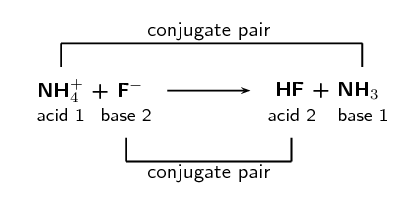
\(\text{NH}_{4}^{+}\text{(aq)} + \text{F}^{-}\text{(aq)} \rightarrow
\text{HF}\text{(aq)} + \text{NH}_{3}\text{(aq)}\)
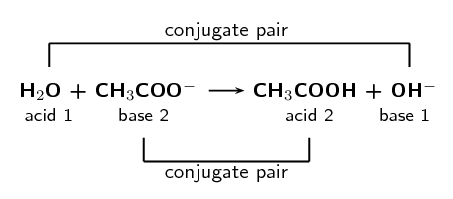
\(\text{H}_{2}\text{O (l)} + \text{CH}_{3}\text{COO}^{-}\text{(aq)} \rightarrow
\text{CH}_{3}\text{COOH (aq)} + \text{OH}^{-}\text{(aq)}\)
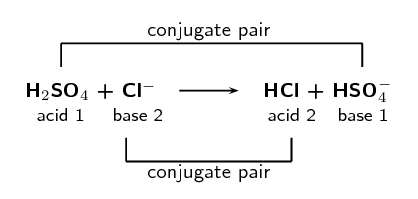
\(\text{H}_{2}\text{SO}_{4}\text{(aq)} + \text{Cl}^{-}\text{(aq)} \rightarrow
\text{HCl (aq)} + \text{HSO}_{4}^{-}\text{(aq)}\)
Write down which reactant is the base and which is the acid.
We break the reaction into two parts:
\(\text{H}_{2}\text{O (aq)} \rightarrow \text{OH}^{-}\text{(aq)}\) and
\(\text{NH}_{3}\text{(aq)} \rightarrow \text{NH}_{4}^{+}\text{(aq)}\)
From this we see that the Bronsted-Lowry acid is \(\text{H}_{2}\text{O}\) and the
Bronsted-Lowry base is \(\text{NH}_{3}\).
Label the conjugate acid-base pairs.
In your own words explain what is meant by the term conjugate acid-base pair.
A conjugate acid-base pair is a reactant and product pair that is transformed into
each other through the loss or gain of a proton. So for example an acid loses a
proton to form a base. The acid and the resulting base are said to be a conjugate
acid-base pair.