3.2 The kinetic molecular theory
|
Previous
3.1 States of matter
|
Next
Chapter summary
|
3.2 The kinetic molecular theory (ESAAL)
The kinetic theory of matter helps us to explain why matter exists in different phases (i.e. solid, liquid and gas), and how matter can change from one phase to another. The kinetic theory of matter also helps us to understand other properties of matter.
Broadly, the kinetic theory of matter says that all matter is composed of particles which have a certain amount of energy which allows them to move at different speeds depending on the temperature (energy). There are spaces between the particles and also attractive forces between particles when they come close together.
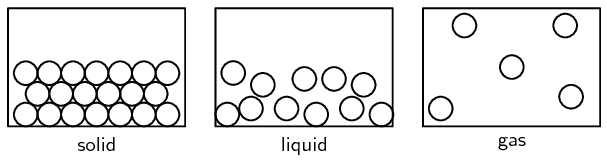
Figure 3.4: The three states of matter
Table 3.1 summarises the characteristics of the particles that are in each phase of matter.
|
Property of matter |
Solid |
Liquid |
Gas |
|
Particles |
Atoms or molecules |
Atoms or molecules |
Atoms or molecules |
|
Energy and movement of particles |
Low energy - particles vibrate around a fixed point. |
Particles have more energy than in the solid phase but less than in the gas phase. |
Particles have high energy and are constantly moving. |
|
Spaces between particles |
Very little space between particles. Particles are tightly packed together. |
Bigger spaces than in solids but smaller than in gases. |
Large spaces because of high energy. |
|
Attractive forces between particles. |
Very strong forces. Solids have a fixed volume. |
Weaker forces than in solids, but stronger forces than in gases. |
Weak forces because of the large distance between particles. |
|
Changes in phase. |
Solids become liquids or gases if their temperature is increased. |
A liquid becomes a gas if its temperature is increased. A liquid becomes a solid if its temperature decreases. |
In general a gas becomes a liquid or solid when it is cooled. Particles have less energy and therefore move closer together so that the attractive forces become stronger, and the gas becomes a liquid or a solid. |
Taking copper as an example we find that in the solid phase the copper atoms have little energy. They vibrate in fixed positions. The atoms are held closely together in a regular pattern called a lattice. If the copper is heated, the energy of the atoms increases. This means that some of the copper atoms are able to overcome the forces that are holding them together, and they move away from each other to form liquid copper. This is why liquid copper is able to flow, because the atoms are more free to move than when they were in the solid lattice. If the liquid is heated further, it will become a gas. Gas particles have lots of energy and are far away from each other. That is why it is difficult to keep a gas in a specific area! The attractive forces between the particles are very weak. Gas atoms will fill the container they are in. Figure 3.1 shows the changes in phase that may occur in matter, and the names that describe these processes.
The three phases of water
Water can be in the form of steam, water liquid or ice. Use marbles (or playdough or clay) to represent water molecules. Arrange the marbles to show the three phases of water. Discuss the properties of each of the phases and the processes and energy in changing from the one phase to the other.


|
Previous
3.1 States of matter
|
Table of Contents |
Next
Chapter summary
|
