What trend is visible when comparing the melting points of monomers and their polymers?
Polymerisation increases the melting point OR while the monomers are in the gas or liquid phase the polymers are solid.
|
Previous
4.6 Addition, elimination and substitution reactions
|
Next
4.8 Chapter summary
|
Polymers are large molecules (macromolecules) that are made up of many repeating structural units called monomers which have various functional groups. To put it more simply, a monomer is like a building block.
Mono means one, while poly means many. So a monomer is the single unit, and a polymer is made from many monomers.
A monomer is a small molecule that can be combined through a reaction to form a polymer. It is a repeating unit in the polymer.
When lots of monomers are joined together by covalent bonds, they form a polymer.
Polymer is a term used to describe large molecules consisting of repeating structural units (monomers) connected by covalent chemical bonds.
A polymer consists of a backbone, made up of repeating units.
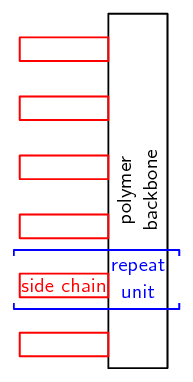
In an organic polymer, the monomers are joined by the carbon atoms of the polymer backbone or chain. A polymer can also be inorganic, in which case there may be atoms such as silicon in the place of carbon atoms. We will look solely at organic polymers. Polymers are a specific group of macromolecules. A macromolecule is any compound with a large number of atoms. A biological macromolecule is one that is found in living organisms. Biological macromolecules include molecules such as carbohydrates, proteins, nucleic acids and lipids. They are essential for all known forms of life to survive.
A macromolecule is any molecule containing a large number of atoms.
The key feature that makes a polymer different from other macromolecules is the repetition of identical or similar monomers in the polymer chain. Polymers will contain chains of the same type of functional group and that functional group is dependent on the monomer used. The examples given over the following pages will help to make these concepts clearer. Plastics are a group of polymers that can be molded during manufacture. They can be one polymer or a blend (mixture) of polymers and may contain other substances as well. These other substances can be inorganic (e.g. used for electronic packaging) or stabilising (e.g. used for increasing fire resistance).
A thermoplastic is a plastic that can be heated and molded multiple times without changing chemically.

The learners should come back to the What is a polymer? concept map throughout this section, whenever they have new information to add. This can then be used as a study tool.
SASOL polymers is the largest division of the Johannesburg based companies' chemical business. Divide the class into 4 groups and have each group research the production of polymers in South Africa, including investigations into one of the products listed below:
Kevlar and Mylar
Windscreens
Break pads
Polymers to replace glass
Research the functions of the materials, who invented or discovered them, and what they are made of. Present your findings to the rest of the class in a five minute discussion.
Read this section (What is a polymer?) carefully. Research any concepts you don't understand and draw up a concept map (mindmap) describing what a polymer is.
A concept map is a way of showing concepts and linking knowledge using graphics. An example is given below:
Polymers are formed through a process called polymerisation, where monomers react together to form a polymer chain. Two of the types of polymerisation reactions are addition polymerisation and condensation polymerisation.
Polymerisation is a process of bonding monomers, or single units together to form longer chains called polymers.
In this type of reaction, monomer molecules are added to a growing polymer chain one at a time. (No small molecules are eliminated in the process).
Four major examples of addition polymers are polyethylene, polypropylene, polyvinylchloride (PVC) and polystyrene (Table 4.18). All four of these organic polymers are also plastics.
|
Polymer name |
Production (metric tons) |
Some uses |
||
|
polyethylene |
\(> 80\) million |
plastic bags |
bottles |
plastic films |
|
polypropylene |
\(> 45\) million |
labelling |
textiles |
stationery |
|
polyvinyl chloride |
\(> 30\) million |
construction |
clothing |
insulation |
|
polystyrene |
\(> 2\) million |
packaging |
molds |
cutlery |
Table 4.18: Common uses of four major polymers formed through addition reactions.
Polyethene (polyethylene)

Figure 4.94: A collection of products made from polyethylene: plastic bags, a syringe, pipes, a plastic tree guard.
Earlier in this chapter we looked at the structure of a group of hydrocarbons called the alkenes. One example is the molecule ethene. The structural formula of ethene is shown in Figure 4.95. When lots of ethene molecules bond together, a polymer called polyethene (commonly called polyethylene) is formed. Ethene is the unsaturated monomer which, when joined to other ethene molecules through an addition reaction, forms the saturated polymer polyethene.
Polyethene is the most common plastic with over 80 million metric tons produced each year. It is commonly known as polyethylene. It is cheap and is used to make squeeze bottles, plastic bags, films, toys and molded objects as well as electric insulation. It has a recycling number 4 which means that it is easy to process, has strength, toughness, flexibility, is easy to seal and has a barrier to moisture.
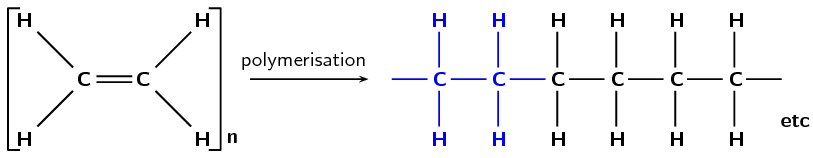
Figure 4.95: The polymerisation of an ethene monomer to form a polyethene polymer. The repeat unit is highlighted in blue.
A polymer may be a chain of thousands of monomers, and so it is impossible to draw the entire polymer. Rather, the structure of a polymer can be condensed and represented as shown in Figure 4.96. The monomer is enclosed in brackets and the n represents the number of repeating units (the saturated form of the monomer) in the polymer, where n is any whole number. What this shows is that the monomer is repeated an indefinite number of times in a molecule of polyethene.
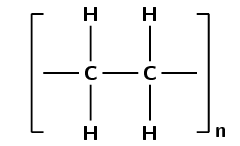
Figure 4.96: A simplified representation of polyethene.
Polypropene (polypropylene)

Figure 4.97: A collection of products made from polypropylene: a lamp cover, computer parts, and shopping trolleys.
Another example of a polymer is polypropene (Figure 4.98). Polypropene (commonly known as polypropylene) is also a plastic, but is stronger than polyethene and is used to make crates, fibres and ropes as well as being used in textiles, stationery and car parts. In this polymer, the monomer is the alkene called propene.
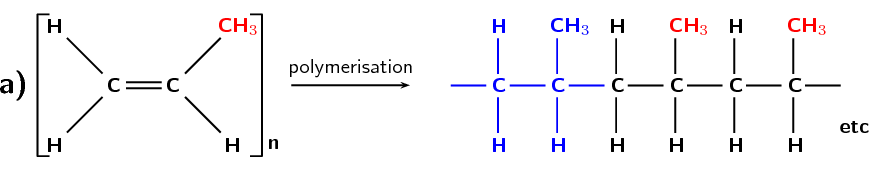
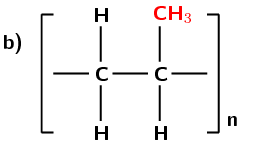
Figure 4.98: (a) The polymerisation of a propene monomer to form a polypropylene polymer. The repeat unit is highlighted in blue. (b) A simplified representation of polypropylene.
Polyvinyl chloride (PVC)

Figure 4.99: A collection of products made from polyvinyl chloride: pipes, electrical tape, and car parts.
Polyvinyl chloride or PVC (Figure 4.100) is formed from the monomer chloroethene, which is commonly known as vinyl chloride. PVC is used in construction, especially plastic piping. With the addition of a plasticiser it is also used in clothing and upholstery and to replace rubber. The role of the plasticiser is to increase the ability of a material to change shape without breaking.
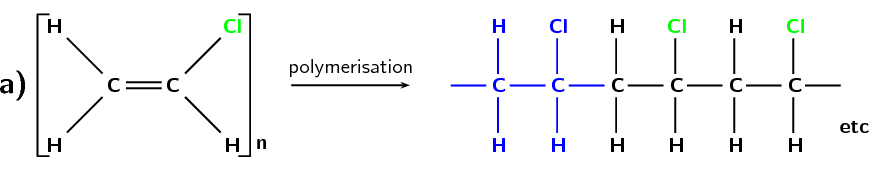

Figure 4.100: (a) The polymerisation of a chloroethane monomer to form a polyvinyl chloride polymer. The repeat unit is highlighted in blue. (b) A simplified representation of polyvinyl chloride.
Polyvinyl acetate
Figure 4.101: Glowing slime made with polyvinyl acetate.
Polyvinyl acetate or PVA (Figure 4.102) is formed from the monomer ethenyl ethanoate, which is commonly known as vinyl acetate. PVA is used in various glues and adhesives (such as wood glue).
Ethenyl is not the same as ethyl. Ethyl is an ethane molecule bonded to another compound (\(\color{red}{\textbf{R}}\)). Ethenyl is an ethene molecule bonded to another compound (\(\color{red}{\textbf{R}}\)).


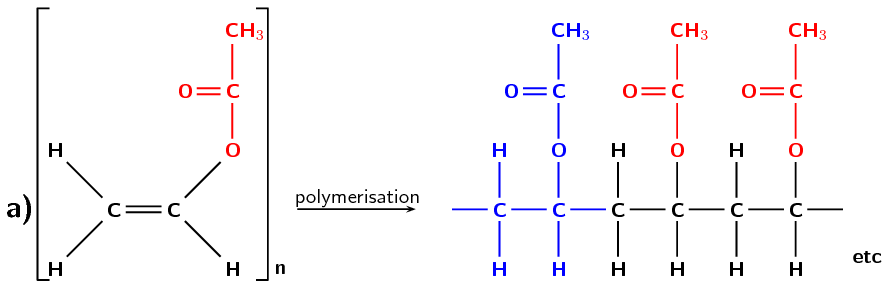
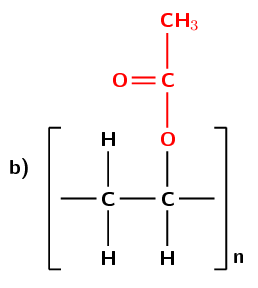
Figure 4.102: (a) The polymerisation of an ethenyl ethanoate monomer to form a polyvinyl acetate polymer. The repeat unit is highlighted in blue. (b) A simplified representation of polyvinyl acetate.
Polystyrene

Figure 4.103: A collection of products made from polystyrene: a cup, a guitar case, polystyrene carvings, and floating pool noodles.
Polystyrene is made from the monomer styrene which is a liquid petrochemical. Styrene consists of a benzene ring (a six membered ring with three double bonds) bonded to an ethene chain. Polystyrene is an aromatic polymer and has many uses including protective packaging, in trays, as plastic lids and bottles.
Benzene can be represented with three double bonds:
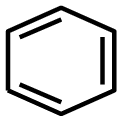
or with a circle inside the ring:
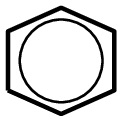
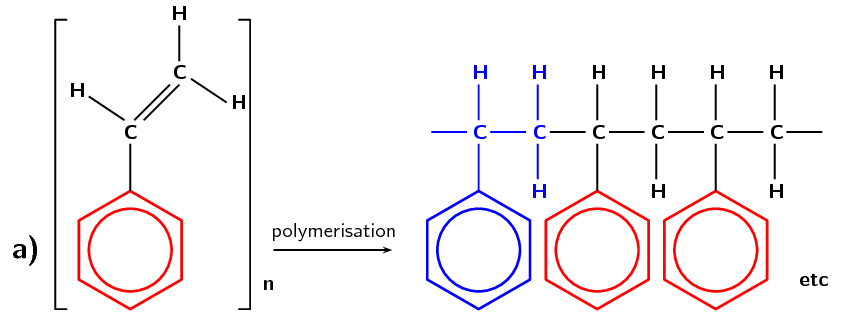
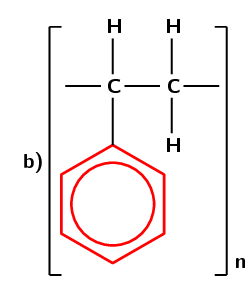
Figure 4.104: (a) The polymerisation of a styrene monomer to form a polystyrene polymer. The repeat unit is highlighted in blue. (b) A simplified representation of polystyrene.
It is interesting to note that the polymerisation of a monomer leads to different physical properties of the polymer (Table 4.19). For the polymers the melting points are dependent on the polymer grade.
Remember that the IUPAC name of:
ethylene is ethene
propylene is propene
vinyl chloride is chloroethene
|
Name |
Melting point (℃) |
Boiling point (℃) |
Phase (at 25℃) |
|
ethylene |
\(-\text{169,2}\) |
\(-\text{103,7}\) |
gas |
|
polyethylene |
\(\text{105}\) - \(\text{130}\) |
does not boil |
solid |
|
propylene |
\(-\text{185,2}\) |
\(-\text{47,6}\) |
gas |
|
polypropylene |
\(\text{130}\) - \(\text{171}\) |
does not boil |
solid |
|
vinyl chloride |
\(-\text{153,8}\) |
\(-\text{13,4}\) |
gas |
|
polyvinyl chloride |
\(\text{100}\) - \(\text{260}\) |
does not boil |
solid |
|
vinyl acetate |
\(-\text{93}\) |
\(\text{72,7}\) |
liquid |
|
polyvinyl acetate |
\(\text{60}\) |
does not boil |
solid |
|
styrene |
\(-\text{30}\) |
\(\text{145}\) |
liquid |
|
polystyrene |
~\(\text{240}\) |
does not boil |
solid |
Table 4.19: Different physical properties of addition monomers and polymers.
Polyethene was discovered by accident, twice. Look into the history of this commonly used plastic as well as other addition polymers.
Who discovered them?
How were they discovered?
What difference have they made to modern life?
Using atomic model kits, jelly tots, or playdough and toothpicks build four ethene monomers, four propene monomers and four vinyl chloride monomers.
Join three of each type of monomer to form polyethene, polypropene and polyvinyl chloride.
Identify the repeating unit in each polymer.
List the differences and similarities between the monomers and the repeating unit.
Note that the monomers all contain double bonds, while the repeating unit has only a single bond.
What trend is visible when comparing the melting points of monomers and their polymers?
Polymerisation increases the melting point OR while the monomers are in the gas or liquid phase the polymers are solid.
Name the monomer (where polymer is given) or polymer (where monomer is given) for the following:
The polymer is polypropene or polypropylene.
The monomer is ethene or ethylene.
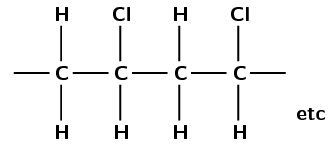
The monomer is chloroethene or vinyl chloride.
In this type of reaction, two monomer molecules combine by means of a covalent bond, and a small molecule such as water is lost in the bonding process. Nearly all biological macromolecules are formed using this process. Polyesters are polymers that form through condensation polymerisation.
Polyesters have a number of characteristics which make them very useful. They are resistant to stretching and shrinking, they are easily washed and dry quickly, and they are resistant to mildew. It is for these reasons that polyesters are being used more and more in textiles. Polyesters are stretched out into fibres and can then be made into fabric and articles of clothing. In the home, polyesters are used to make clothing, carpets, curtains, sheets, pillows and upholstery.
Polyesters are a group of polymers that contain the ester functional group in their main chain. This bond is called an ester linkage. For a polyester however there needs to be continuation of the chain. This requires a -diol (two alcohol functional groups) and a diacid (two carboxylic acid functional groups) as shown in Figure 4.105, or a monomer that contains both a hydroxyl group and a carboxylic acid.
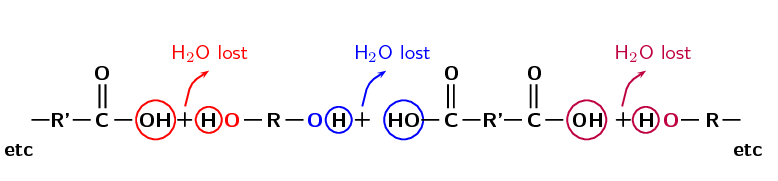
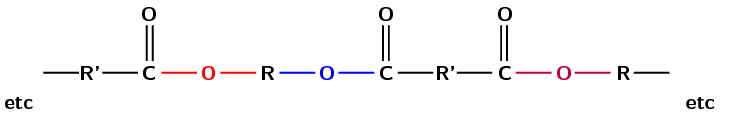
Figure 4.105: The propagation of the ester linkage in a polyester.
Polyethylene terephthalate (PET)

Figure 4.106: A collection of products made from polyethylene terephthalate: sailcloth and plastic squeeze bottles.
Although there are many forms of polyesters, the term polyester usually refers to polyethylene terephthalate (PET). PET is made from ethan-1,2-diol (ethylene glycol, an alcohol) and terephthalic acid (a carboxylic acid). In the reaction, a hydrogen atom is lost from the alcohol, and a hydroxyl group is lost from the carboxylic acid. Together these form one water molecule which is lost during condensation reactions. A new bond is formed between an oxygen and a carbon atom. This bond is called an ester linkage. The reaction is shown in Figure 4.107.
Polyethylene terephthalate (PET) is not just a textile. PET is in fact a plastic which can also be used to make plastic drink bottles. Many drink bottles are recycled by being reheated and turned into polyester fibres. This type of recycling helps to reduce disposal problems.
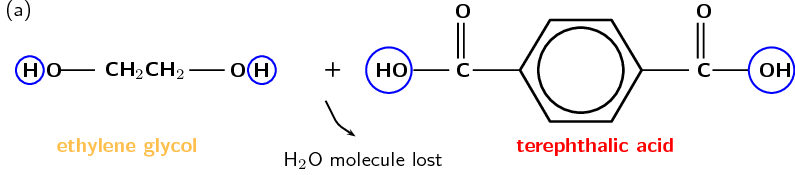
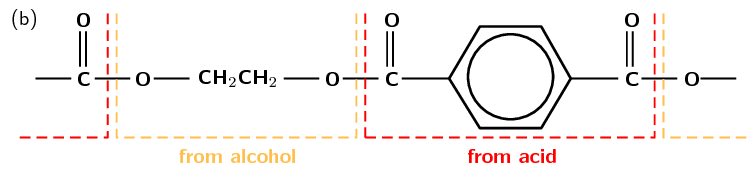
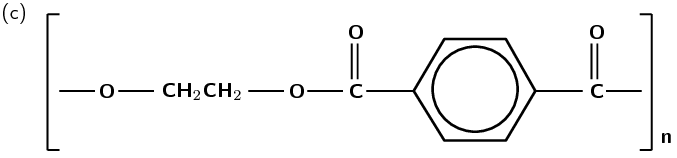
Figure 4.107: (a) Ethylene glycol (alcohol) and terephthalic acid (carboxylic acid) monomers react to form (b) the polymer poly(ethylene terephthalate) (PET). (c) A simplified representation of PET.
Polylactic acid (PLA)
3D printing is the process of printing a solid, three-dimensional object. If you have a 3D printer it is possible to print objects ranging from fashion items (e.g. cell phone covers), to human organs, although organs require living cells as the 'ink'.

Figure 4.108: 3-D printing using polylactic acid as the ink.
Despite the name, polylactic acid (PLA) is actually a polyester (look for the ester linkage we spoke about before). It is an interesting polymer because the monomer used for this polymer (Figure 4.109) comes from the biological fermentation of plant materials, while most monomers used in plastics come from petroleum. As a result PLA is biodegradable and has low carbon dioxide (\(\text{CO}_{2}\)) emissions.
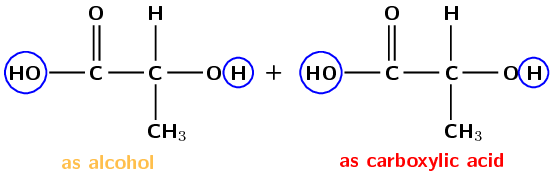
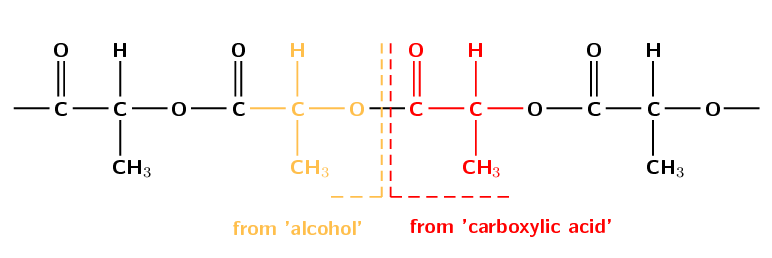
Figure 4.109: Ester linkages between lactic acid monomers.
PLA is mostly used for packaging material and, because it is biodegradable, it has the potential to alleviate land-fill disposal problems. The PLA polymer structural repeat unit is given in Figure 4.110.
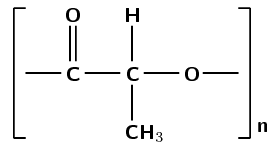
Figure 4.110: The polyester polylactic acid.
|
Polymer name |
Some uses |
||
|
polyethylene terephthalate |
synthetic fibre |
plastic containers |
resins |
|
polylactic acid |
medical implants |
packaging |
textiles |
Table 4.20: Some common uses of polymers formed through condensation reactions.
For polyethylene terephthalate there are two monomers ethylene glycol and terephthalic acid that join to form the polymer. Each monomer has its own unique properties (Table 4.21).
|
Name |
Monomer/ polymer |
Melting point (℃) |
Boiling point (℃) |
Phase (at 25℃) |
|
\(\color{blue}{\text{terephthalic acid}}\) |
monomer |
\(\text{300}\) |
sublimes (solid \(\to\) gas) |
solid |
|
\(\color{blue}{\text{ethylene glycol}}\) |
monomer |
\(-\text{12,9}\) |
\(\text{197,3}\) |
liquid |
|
\(\color{blue}{\text{polyethylene}}\) \(\color{blue}{\text{terephthalate}}\) |
polymer |
\(>\text{250}\) |
decomposes |
solid |
|
\(\color{red}{\text{lactic acid}}\) |
monomer |
\(\text{16,8}\) |
\(\text{122}\) |
liquid |
|
\(\color{red}{\text{polylactic acid}}\) |
polymer |
\(\text{150}\) - \(\text{160}\) |
does not boil |
solid |
Table 4.21: Different physical properties of condensation monomers and polymers.
What is the main difference between the reactants used in an addition polymerisation and those used in a condensation polymerisation?
A condensation reaction requires two monomer molecules (or a monomer with two functional groups) while an addition reaction requires only one.
OR
An addition polymerisation is an addition reaction while a condensation polymerisation is a substitution reaction.
OR
An addition polymerisation requires an unsaturated monomer while a condensation polymerisation does not.
There are three stages in the process of addition polymerisation. Initiation refers to a chemical reaction that triggers the first reaction. In other words, initiation is the starting point of the polymerisation reaction. Chain propagation is the part where monomers are continually added to form a longer and longer polymer chain. During chain propagation it is the reactive end groups of the polymer chain that react in each propagation step to add a new monomer to the chain. Once a monomer has been added the reactive part of the polymer is now in this last monomer unit so that propagation will continue. Termination refers to a chemical reaction that destroys the reactive part of the polymer chain so that propagation stops.
It is important to be able to identify the monomer used to produce a polymer from the repeat unit of the chain.
Which monomer was used to make this polymer (give the name and draw the structure):
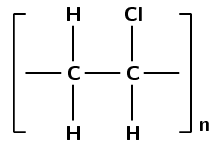
This polymer contains a chlorine atom. Which polymers contain chlorine? Polyvinyl chloride contains chlorine.
PVC forms through an addition reaction. There are two carbon atoms in the repeat unit (ethane) and the polymer contains chlorine atoms.
An addition reaction indicates that the monomer must contain a double bond. As the polymer contains units of ethane the monomer must be an ethene. The monomer must contain a chlorine atom.
The monomer must be chloroethene also known as vinyl chloride
Which monomer was used to make this polymer (give the name and draw the structure):
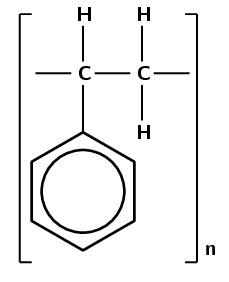
This polymer contains a benzene ring connected to two carbon atoms. Which polymers contain a benzene ring? Polystyrene contains a benzene ring connected to two carbon atoms.
Polystyrene forms through an addition reaction. There are two carbon atoms attached to the benzene ring.
An addition reaction means that the monomer must contain a double bond. The polymer contains two carbon atoms attached to the benzene ring which must be connected by a double bond in the monomer.
The monomer must be styrene.
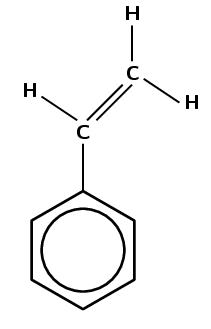
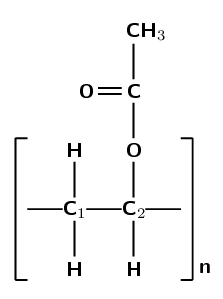
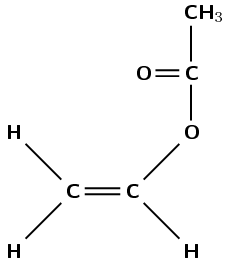
Give the structure of the monomer used to make polyvinyl acetate:
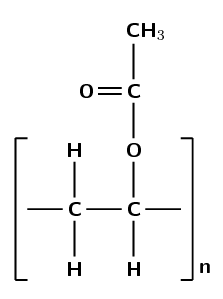
Polyvinyl acetate must form from vinyl acetate. The IUPAC name of vinyl acetate is ethenyl ethanoate. The polymer forms through an addition reaction.
An addition reaction means that the monomer must contain a double bond. The only place this bond can occur is between carbon 1 and carbon 2:
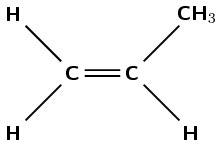
Which polymer is formed from propene? Give the name and repeat structural unit.
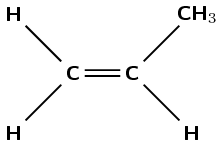
Propene contains a double bond between the first and second carbon atoms.
The polymer must form through an addition reaction by breaking the double bond. There must be a methyl group on every second carbon atom.
The polymer must be polypropene (polypropylene).
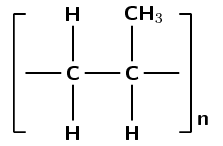
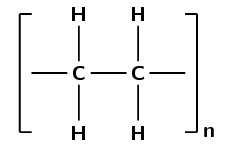
Was an addition or a condensation reaction used to make this polymer?
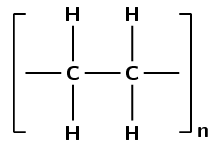
There are only single carbon-carbon bonds, there are no functional groups.
As there are no oxygen atoms in the repeat unit, this polymer could only have formed from a monomer containing a double bond.
This polymer must have formed through an addition reaction.
(This is polyethene and was formed by the addition of ethene monomers)
Was an addition or a condensation reaction used to make this polymer?
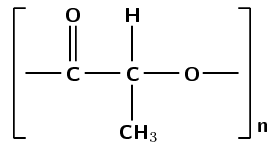
There are carbon-carbon single bonds. If you expand the repeating unit you can see that there is an ester linkage in the structural chain.
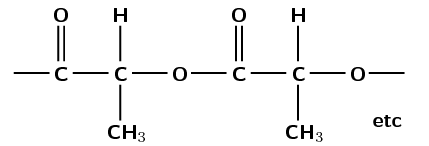
The ester linkage requires an alcohol and a carboxylic acid monomer or a monomer that contains both a hydroxyl and carboxyl functional group.
The reaction of an alcohol and carboxylic acid to form an ester linkage (and resulting in the loss of water) is a condensation reaction.
(This is polylactic acid and was formed by the condensation reaction of lactic acid monomers)
In the making polymer slime experiment make sure the learners keep the slime away from their clothes as it can permanently stain. You can store the slime by dipping it in some water and then storing it in an air-tight container. However, it may become mouldy after a few days, at that stage throw it away (not down the sink).
To compare the differences in slime made from polyvinyl alcohol and polyvinyl acetate.
4% aqueous polyvinyl alcohol solution, 4% aqueous borax (hydrated sodium tetraborate) solution, white glue (polyvinyl acetate), food colourant
a beaker, two disposable plastic cups, a metal spatula, disposable plastic gloves
Place \(\text{40}\) \(\text{cm$^{3}$}\) of the polyvinyl alcohol solution in one plastic cup and label it A.
Place \(\text{20}\) \(\text{cm$^{3}$}\) of the white glue in the second cup. Add \(\text{20}\) \(\text{cm$^{3}$}\) of water and stir. Label this cup B.
Add one drop of food colourant to each cup and stir well.
Measure out \(\text{10}\) \(\text{cm$^{3}$}\) of the borax solution into the beaker, then add to cup A.
Repeat step 4 with cup B.
Stir until gelling is complete (i.e. the slime).
Put on the disposable plastic gloves, take the slime out of cup A and roll it around in your hand to remove air bubbles, repeat with the slime in cup B.
Pull each slime apart slowly, what happens?
Pull each slime apart quickly, what happens?
Roll each slime into a ball and drop it onto the bench, what happens?
Place a small bit of each slime on the bench and hit it hard with your hand, what happens?
What are the similarities and differences between the two slimes?
You should find that the slime does not break under slow stress, but will break if you pull it apart too quickly. This is because the slime can expand under stress. It should even bounce slightly if you throw it onto a hard surface.
The Great Pacific Garbage Patch is a large area of waste that has collected in the central North Pacific Ocean. It is estimated to cover at least \(\text{700 000}\) \(\text{km$^{2}$}\).

Although plastics have had a huge impact globally, there is also an environmental price that has to be paid for their use. The following are just some of the ways in which plastics can cause damage to the environment.
Waste disposal
Plastics are not easily broken down by micro-organisms and therefore most are not easily biodegradeable. This leads to waste disposal problems.
Air pollution
When plastics burn, they can produce toxic gases such as carbon monoxide, hydrogen cyanide and hydrogen chloride (particularly from PVC and other plastics that contain chlorine or nitrogen).
Recycling
It is difficult to recycle plastics because each type of plastic has different properties and so different recycling methods may be needed for each plastic. Some plastics can be remelted and re-used, while others can be ground up and used as a filler. One of the problems with recycling plastics is that they have to be sorted according to plastic type. A list of some of the different types and their identification codes are given in Table 4.22. Alternatively, plastics should be re-used. In many countries, including South Africa, shoppers must now pay for plastic bags. This encourages people to collect and re-use the bags they already have.
|
PIC |
Plastic type |
Properties |
Uses |
Recycling |

|
polyethylene terephthalate |
very clear high strength barrier to gas barrier to moisture |
water bottles peanut butter jars mouthwash bottles |
can recycle most places |

|
high-density polyethylene |
very stiff high strength barrier to moisture allows gas through |
yoghurt tubs detergent bottles cereal box liners |
can recycle most places |

|
polyvinyl chloride |
can be blended easily high strength very toughness |
detergent bottles medical equipment piping |
very rarely recycled |

|
low-density polyethylene |
very flexible high strength barrier to moisture |
clothing squeeze bottles bread bags |
not often recycled |
|
polypropylene |
high strength resistance to heat barrier to moisture |
syrup bottles plastic caps straws |
fairly easy to recycle |
|
polystyrene |
many uses very clear is easily formed |
egg cartons disposable cups take-away boxes |
fairly easy to recycle |
|
other |
dependent on combination of polymers |
large containers sunglasses computer cases |
often not recycled |
Table 4.22: The plastic identification code (PIC), type, properties and uses of various commonly used plastics.
Read the following extract, taken from Planet Ark (September 2003), and then answer the questions that follow.
'South Africa launches a programme this week to exterminate its national flower - the millions of used plastic bags that litter the landscape.
Beginning on Friday, plastic shopping bags used in the country must be both thicker and more recyclable, a move officials hope will stop people from simply tossing them away. 'Government has targeted plastic bags because they are the most visible kind of waste,' said Phindile Makwakwa, spokeswoman for the Department of Environmental Affairs and Tourism. 'But this is mostly about changing people's mindsets about the environment.'
South Africa is awash in plastic pollution. Plastic bags are such a common eyesore that they are dubbed roadside daisies and referred to as the national flower. Bill Naude of the Plastics Federation of South Africa said the country used about eight billion plastic bags annually, a figure which could drop by 50 percent if the new law works.'
It is difficult sometimes to imagine exactly how much waste is produced in our country every year. Where does all of this go to? You are going to do some simple calculations to try to estimate the volume of plastic packets that is produced in South Africa every year.
Take a plastic shopping packet and fold it until it forms a tightly compressed cube.
Measure the approximate length, breadth and depth of your compressed plastic bag.
Calculate the approximate volume that is occupied by the packet.
Now calculate the approximate volume of your classroom by measuring its length, breadth and height.
Calculate the number of compressed plastic packets that would fit into a classroom of this volume.
If South Africa produces an average of 8 billion plastic bags each year, how many classrooms would be filled if all of these bags were thrown away and not re-used?
What has South Africa done to try to reduce the number of plastic bags that are produced?
Do you think this has helped the situation?
What can you do to reduce the amount of plastic that you throw away?
Take a survey of your classmates, family and friends on the quantities and types of solid waste they generate. What methods could they use to reduce that waste?
It has now been over ten years since shops started charging for plastic bags. Research the effects this law has had on South African plastic waste.
Read the following extract, taken from Nova: Science in the news (July 2006), and then answer the questions that follow.
Our whole world seems to be wrapped in plastic. Almost every product we buy, most of the food we eat and many of the liquids we drink come encased in plastic. Plastic packaging provides excellent protection for the product, it is cheap to manufacture and seems to last forever. Lasting forever, however, is proving to be a major environmental problem. Another problem is that traditional plastics are manufactured from non-renewable resources - oil, coal and natural gas. In an effort to overcome these problems, researchers and engineers have been trying to develop biodegradable plastics that are made from renewable resources, such as plants.
The term biodegradable means that a substance can be broken down into simpler substances by the activities of living organisms, and therefore is unlikely to remain in the environment. The reason most plastics are not biodegradable is because their long polymer molecules are too large and too tightly bonded together to be broken apart and used by decomposer organisms. However, plastics based on natural plant polymers that come from wheat or corn starch have molecules that can be more easily broken down by microbes.
Starch is a natural polymer. It is a white, granular carbohydrate produced by plants during photosynthesis and it serves as the plant's energy store. Many plants contain large amounts of starch. Starch can be processed directly into a bioplastic but, because it is soluble in water, articles made from starch will swell and deform when exposed to moisture, and this limits its use. This problem can be overcome by changing starch into a different polymer. First, starch is harvested from corn, wheat or potatoes, then micro-organisms transform it into lactic acid, a monomer. Finally, the lactic acid is chemically treated to cause the molecules of lactic acid to link up into long chains or polymers, which bond together to form a plastic called polylactide (PLA).
PLA can be used for products such as plant pots and disposable nappies. It has been commercially available in some countries since 1990, and certain blends have proved successful in medical implants, sutures and drug delivery systems because they are able to dissolve away over time. However, because PLA is much more expensive than normal plastics, it has not become as popular as one would have hoped.
In your own words, explain what is meant by a biodegradable plastic.
Using your knowledge of chemical bonding, explain why some polymers are biodegradable and others are not.
Explain why lactic acid is a more useful monomer than starch, when making a biodegradable plastic.
If you were a consumer (shopper), would you choose to buy a biodegradable plastic rather than another? Explain your answer.
What do you think could be done to make biodegradable plastics more popular with consumers?
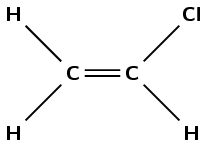
The following monomer is a reactant in a polymerisation reaction:
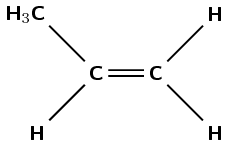
What is the IUPAC name of this monomer?
propene
Give the structural representation of the polymer that is formed in this polymerisation reaction.
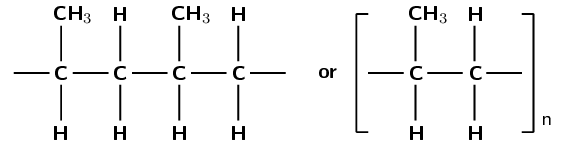
Is the reaction an addition or condensation reaction?
Addition
The polymer below is the product of a polymerisation reaction.
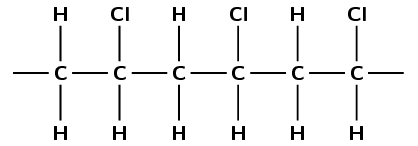
Give the structural formula of the monomer used to form this polymer.
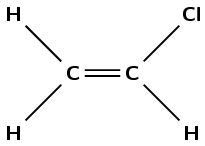
What is the name of the monomer?
The monomer is chloroethene or vinyl chloride.
Draw the abbreviated structural formula for the polymer (the repeat unit).
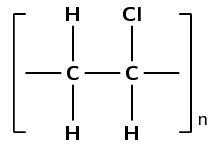
Has this polymer been formed through an addition or condensation polymerisation reaction?
Addition
A polymerisation reaction takes place between two lactic acid monomers.
Give the structural representation for:
lactic acid
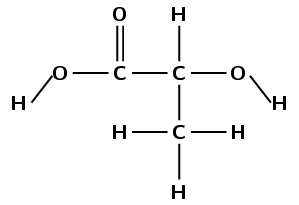
the repeat unit of the polymer product
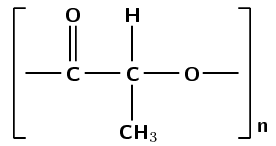
What is the name of the product?
polylactic acid
Was this polymer formed through an addition or a condensation reaction?
Condensation
|
Previous
4.6 Addition, elimination and substitution reactions
|
Table of Contents |
Next
4.8 Chapter summary
|