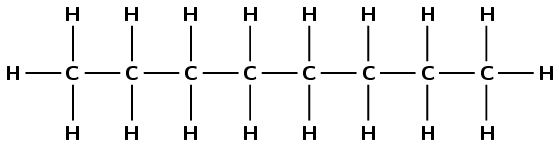
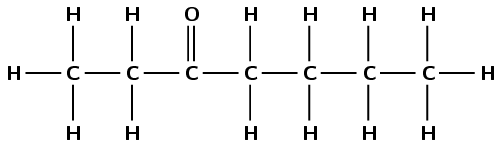
Octane
The suffix -ane tells us that this is an alkane. The prefix oct- tells us that there are eight carbon atoms in the longest chain.
|
Previous
4.2 Organic molecular structures
|
Next
4.4 Physical properties and structure
|
In order to give compounds a name, certain rules must be followed. When naming organic compounds, the IUPAC (International Union of Pure and Applied Chemistry) nomenclature (naming scheme) is used. This is to give consistency to the names. It also enables every compound to have a unique name, which is not possible with the common names used (for example in industry). We will first look at some of the steps that need to be followed when naming a compound, and then try to apply these rules to some specific examples.
A good general rule to follow is to start at the end (the suffix) and work backwards (from right to left) in the name.
Molecules can contain both double or triple bonds and other functional groups (e.g. an alkene and an alcohol functional group in one molecule - propenol). However, all molecules explored in this book will contain only single carbon-carbon bonds when combined with other functional groups.
Recognise the functional group in the compound. This will determine the suffix of the name (see Table 4.5).
|
Functional group |
suffix |
|
alkane |
-ane |
|
alkene |
-ene |
|
alkyne |
-yne |
|
alcohol |
-ol |
|
aldehyde |
-al |
|
ketone |
-one |
|
carboxylic acid |
-oic acid |
|
ester |
-oate |
Table 4.5: The suffix associated with various functional groups.
Find the longest continuous carbon chain that contains the functional group (it won't always be a straight chain) and count the number of carbon atoms in this chain. This number will determine the prefix (the beginning) of the compound's name (see Table 4.6).
|
Carbon atoms |
prefix |
|
1 |
meth- |
|
2 |
eth- |
|
3 |
prop- |
|
4 |
but- |
|
5 |
pent- |
|
6 |
hex- |
|
7 |
hept- |
|
8 |
oct- |
|
9 |
non- |
|
10 |
dec- |
Table 4.6: The prefix of a compound's name is determined by the number of carbon atoms in the longest chain that contains the functional group.
Number the carbons in the longest carbon chain (Important: If the molecule is not an alkane (i.e. has a functional group) you need to start numbering so that the functional group is on the carbon with the lowest possible number). Start with the carbon at the end closest to the functional group.
Look for any branched groups:
If there are no branched groups this step can be ignored.
|
Number |
prefix |
|
2 |
di- |
|
3 |
tri- |
|
4 |
tetra- |
Table 4.7: Prefixes for multiple substituents with the same name. These apply to multiple functional groups as well.
For the alkyl halides the halogen atom is treated in much the same way as branched groups:
|
Halogen |
name |
|
fluorine |
fluoro |
|
chlorine |
chloro |
|
bromine |
bromo |
|
iodine |
iodo |
Table 4.8: Naming halogen atoms in organic molecules.
If there are no halogen atoms this step can be ignored.
Combine the elements of the name into a single word in the following order:
The suffix for an alkane is -ane.
Give the IUPAC name for the following compound:
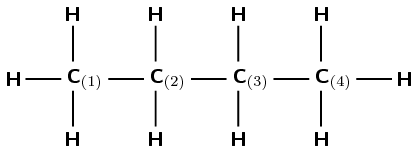
Note: The numbers attached to the carbon atoms would not normally be shown. The carbon atoms have been numbered to help you to name the compound.
The compound is a hydrocarbon with single bonds between the carbon atoms. It is an alkane and will have a suffix of -ane.
There are four carbon atoms in the longest chain. The prefix of the compound will be but-.
The numbering has been done for you here.
There are no branched groups in this compound.
The name of the compound is butane.
Give the IUPAC name for the following compound:
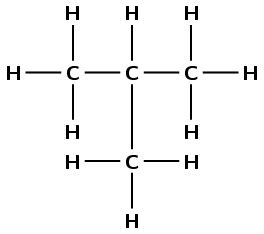
The compound is a hydrocarbon with single bonds between the carbon atoms. It is an alkane and will have the suffix -ane.
There are three carbon atoms in the longest chain. The prefix for this compound is prop-.
If we start at the carbon on the left, we can number the atoms as shown in red (left). If we start at the carbon on the right, we can number the atoms as shown in blue (right).
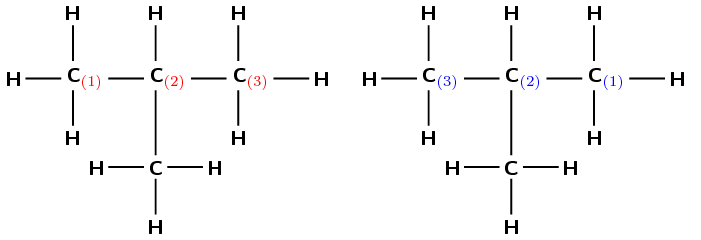
There is a branched group attached to the second carbon atom. In this case the methyl group is on carbon 2 regardless of which side you number the longest chain from.
This group has the formula \(\text{CH}_{3}\), which is methane without a hydrogen atom. However, because it is not part of the main chain, it is given the suffix -yl (i.e. methyl). The position of the methyl group comes just before its name (see the next step).
The compound's name is 2-methylpropane.
Give the IUPAC name for the following compound:
\(\text{CH}_{3}\text{CH}(\text{CH}_{3})\text{CH}(\text{CH}_{3})\text{CH}_{3}\)
(Remember that the side groups are shown in brackets after the carbon atom to which they are attached).
The structural formula of the compound is:
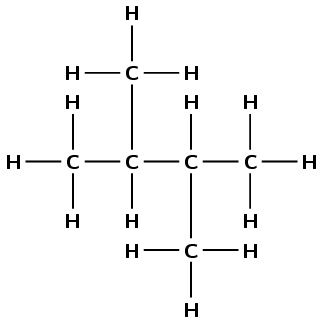
The compound is a hydrocarbon with single bonds between the carbon atoms. It is an alkane and will have the suffix -ane.
There are four carbon atoms in the longest chain. The prefix for this compound is but-.
If we start at the carbon on the left, we can number the atoms as shown in red (left). If we start at the carbon on the right, we can number the atoms as shown in blue (right).
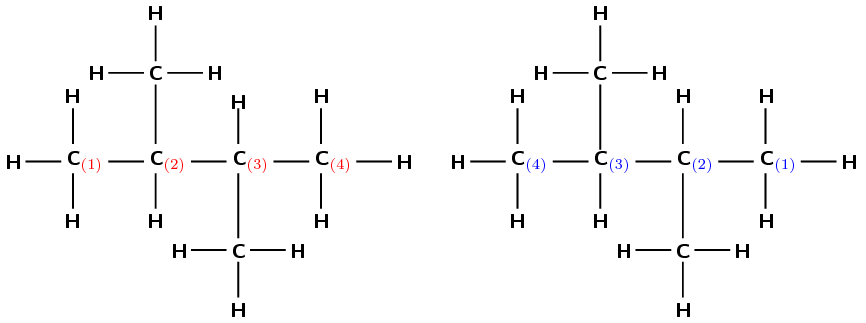
There are two methyl groups attached to the main chain. The first one is attached to the second carbon atom and the second methyl group is attached to the third carbon atom. Notice that in this example it does not matter how you have chosen to number the carbons in the main chain; the methyl groups are still attached to the second and third carbon atoms and so the naming of the compound is not affected.
This group will be 2,3-dimethyl-
The compound's name is 2,3-dimethylbutane.
Give the IUPAC name for the following compound:
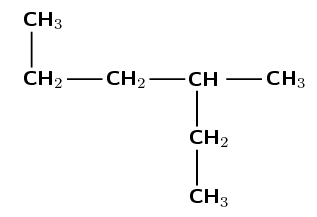
The compound is a hydrocarbon with single bonds between the carbon atoms. It is an alkane and will have the suffix -ane.
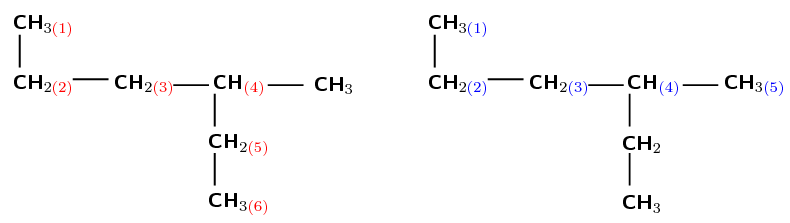
There are six carbons in the longest chain if they are numbered as shown in red (on the left). There are only five carbon atoms if they are numbered as shown in blue (right). Therefore, the red numbering (on the left) is correct and the prefix for the compound is hex-.
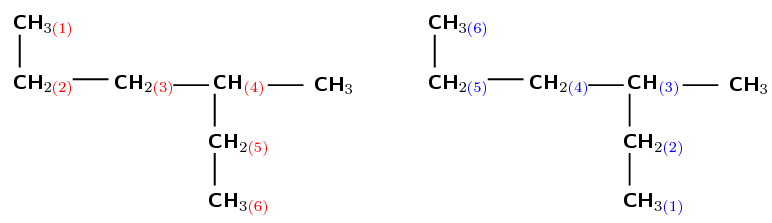
There is one methyl group attached to the main chain. If we number as shown in red (on the left) the methyl is attached to the fourth carbon atom. If we number as shown in blue (on the right) the methyl is attached to the third carbon atom.
After functional groups, the branched groups should have the lowest numbers possible. Therefore the blue numbering (on the right) is correct. The methyl is attached to the third carbon atom (3-methyl).
The compound's name is 3-methylhexane.
Draw the semi-structural structural and condensed structural formula for the organic compound 2,2,4-trimethylhexane
The name ends in -ane therefore the compound is an alkane.
The longest chain has the prefix hex-. There are therefore 6 carbon atoms in the longest chain.
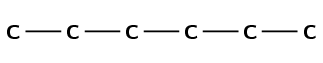
The compound is 2,2,4-trimethylhexane. Therefore there are three branched groups. Two on carbon 2 and one on carbon 4.
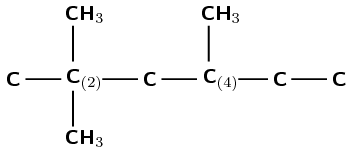
Carbon atoms can have four single bonds. Therefore wherever a carbon atom has less than four bonds draw in hydrogen atoms until there are four bonds.
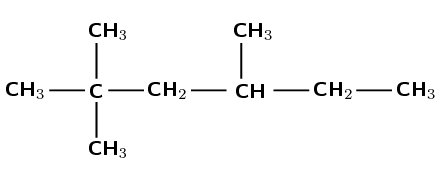
First condense the main chain: \(\text{CH}_{3}\text{CCH}_{2}\text{CHCH}_{2}\text{CH}_{3}\)
Then add the side chains (in brackets) on the relevant carbon atoms:
\(\text{CH}_{3}\text{C}(\text{CH}_{3})_{2}\text{CH}_{2}\text{CH}(\text{CH}_{3})\text{CH}_{2}\text{CH}_{3}\)
Give the structural formula for each of the following alkanes
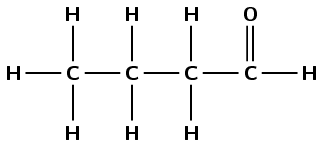
Octane
The suffix -ane tells us that this is an alkane. The prefix oct- tells us that there are eight carbon atoms in the longest chain.

Propane
The suffix -ane tells us that this is an alkane. The prefix prop- tells us that there are three carbon atoms in the longest chain.
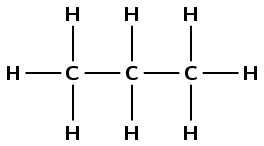
2-methylpropane
The suffix -ane tells us that this is an alkane. The prefix prop- tells us that there are three carbon atoms in the longest chain. 2-methyl tells us that there is a methyl (\(\text{CH}_{3}-\)) branched group attached to the second carbon atom.
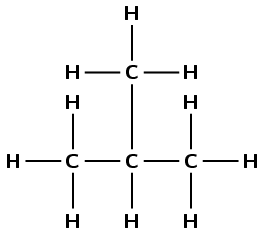
3-ethylpentane
The suffix -ane tells us that this is an alkane. The prefix pent- tells us that there are five carbon atoms in the longest chain. 3-ethyl tells us that there is an ethyl branched group (\(\text{CH}_{3}\text{CH}_{2}-\)) group attached to the third carbon atom.
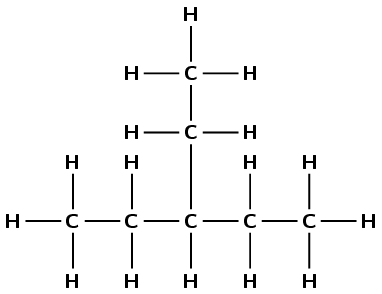
Give the IUPAC name for each of the following alkanes:
\(\text{CH}_{3}\text{CH}_{2}\text{CH}(\text{CH}_{3})\text{CH}_{2}\text{CH}_{3}\)
This is hard to do unless you draw the structural formula of the molecule out. It is recommended that you do this in exams.
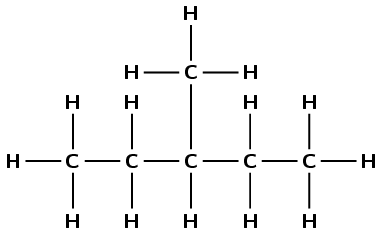
There are five carbons in the longest chain so the prefix is pent-. There are only single carbon-carbon bonds and no other functional group so the compound is an alkane and the suffix is -ane. There is one methyl group at position 3 (you can number from either end of the chain for this example). So the compound is 3-methylpentane.
\(\text{CH}_{3}\text{CH}(\text{CH}_{3})\text{CH}_{2}\text{CH}(\text{CH}_{3})\text{CH}_{3}\)
This is hard to do unless you draw the structural formula of the molecule out. It is recommended that you do this in exams.
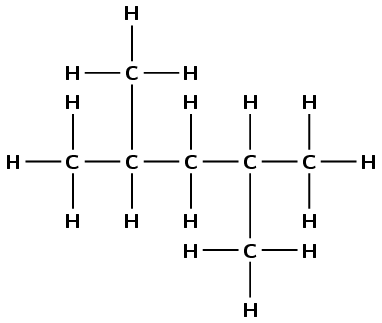
There are five carbons in the longest chain, so the prefix is pent-. There are only single carbon-carbon bonds so the compound is an alkane and the suffix is -ane. There is one methyl group at position 2 and one at position 4 (once again you can number from either end). So the compound is
2,4-dimethylpentane.
\(\text{CH}_{3}\text{CH}_{2}\text{CH}_{2}\text{CH}_{2}\text{CH}_{2}\text{CH}_{3}\)
This is hard to do unless you draw the structural formula of the molecule out. It is recommended that you do this in exams.
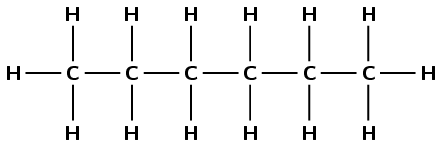
There are six carbon atoms in the longest chain so the prefix is hex-. There are only single carbon-carbon bonds and no other functional group so the compound is an alkane and the suffix is -ane. There are no branched groups, so the molecule is hexane.
\(\text{CH}_{3}\text{CH}_{3}\)
This is hard to do unless you draw the structural formula of the molecule out. It is recommended that you do this in exams.
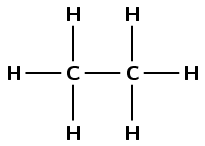
There are two carbon atoms in the longest chain so the prefix is eth-. There are only single carbon-carbon bonds and no other functional group so the compound is an alkane and the suffix is -ane. There are no branched groups, so the molecule is ethane.
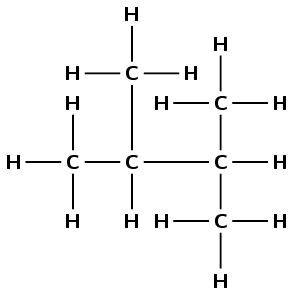
There are four carbon atoms in the longest chain, so the prefix is but-. There are two methyl branches at positions 2 and 3. The functional group is an alkane, so the suffix is -ane. Combining all this information we get: 2,3-dimethylbutane. Note that in this example it does not matter which end you start numbering from.
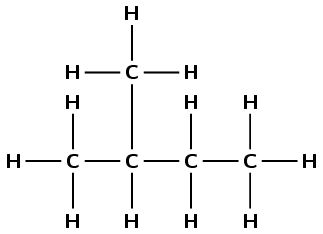
There are four carbon atoms in the longest chain so the prefix is but-. The functional group is an alkane, so the suffix is -ane. There is one branched group which is a methyl group and this is at position 2. The molecule is 2-methylbutane.
Note that in this example it does matter which way you number the chain as the branched group needs to have the lowest number possible and so the compound is not 3-methylbutane.
The suffix for an alkene is -ene.
Give the IUPAC name for the following compound:
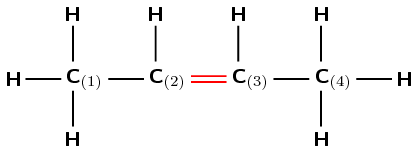
The compound has a double carbon-carbon bond and is an alkene. It will have the suffix -ene.
The functional group is a double bond, so the longest chain must contain the double bond. There are four carbon atoms in the longest chain and so the prefix for this compound will be but-.
Remember that the carbon atoms must be numbered so that the functional group is at the lowest numbered carbon atom possible. In this case, it doesn't matter whether we number the carbons from the left to right, or from the right to left. The double bond will still fall between the second and third carbon atoms.
There are no branched groups in this molecule.
The name of this compound is but-2-ene or 2-butene.
Draw the structural and molecular formula for the organic compound
3-methylbut-1-ene
The suffix -ene means that this compound is an alkene and there must be a double bond in the molecule. The number 1 immediately before the suffix means that the double bond must be at the first carbon in the chain (but-1-ene).
The prefix for the compound is but- so there must be four carbons in the longest chain containing the double bond.
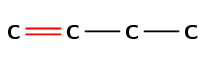
There is a methyl group at the third carbon atom in the chain. Count from the left so that the double bond carbon is the first carbon atom.
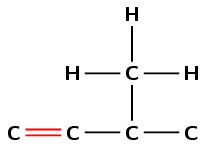
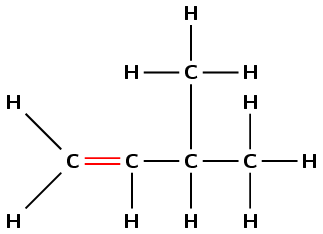
There are 5 carbon atoms and 10 hydrogen atoms so the molecular formula is \(\text{C}_{5}\text{H}_{10}\).
(Remember that there is no structural information given by the molecular formula)
Give the IUPAC name for the following compound:
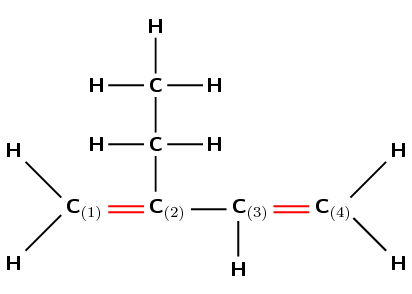
The compound is an alkene and will have the suffix -ene. There is a double bond between the first and second carbons and also between the third and fourth carbons. The organic compound therefore contains '1,3-diene'.
Remember that the main carbon chain must contain both the double bonds. There are four carbon atoms in the longest chain containing the double bonds and so the prefix for this compound will be but-. The carbon atoms are already numbered 1 to 4 in the diagram.
There is an ethyl group on the second carbon.
Note that if we had numbered from the right to left the suffix would still have been 1,3-diene, however the ethyl group would have been on the third carbon. So we had to number left to right.
The name of this compound is 2-ethylbut-1,3-diene.
Give the IUPAC name for each of the following alkenes:
\(\text{CH}_{2}\text{CHCH}_{2}\text{CH}_{2}\text{CH}_{3}\)
Draw the structural representation:
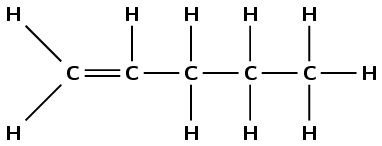
The molecule contains a double carbon-carbon bond. It is an alkene and so the suffix is -ene. There are five carbons in the longest chain, so the prefix is pent-. There are no branched groups. The double bond occurs between carbons 1 and 2. So the molecule is 1-pentene or pent-1-ene.
Note that the way you number the carbon atoms matters here, the molecule is not pent-4-ene.
\(\text{CH}_{3}\text{CHCHCH}_{3}\)
Draw the structural representation:
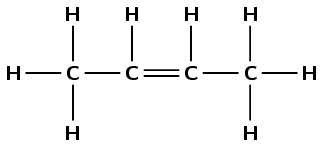
The molecule contains a double carbon-carbon bond. It is an alkene and so the suffix is -ene. There are four carbons in the longest chain so the prefix is but-. There are no branched groups. The double bond occurs between carbons 2 and 3. The molecule is 2-butene or but-2-ene.
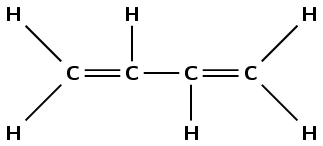
The compound contains two double carbon-carbon bonds. It is an alkene and so the suffix is -diene. There are four carbons in the longest chain containing the double bonds, so the prefix is but-. There are no branched chains. The first double bond occurs between carbons 1 and 2. The second double bond occurs between carbons 3 and 4. The compound is but-1,3-diene.
Give the structural formula for each of the following alkenes:
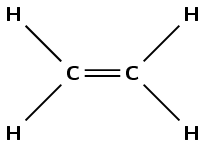
ethene
The prefix eth- tells us there are two carbon atoms in the chain. The suffix -ene tells us there is a double bond between these carbon atoms.
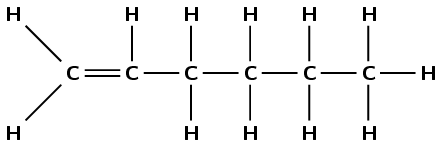
hex-1-ene
The prefix hex- tells us there are six carbon atoms in the chain. The suffix -1-ene tells us there is a double bond between the first and second carbon atoms.
hept-3-ene
The prefix hept- tells us there are seven carbon atoms in the chain. The suffix -3-ene tells us there is a double bond between the third and fourth carbon atoms.
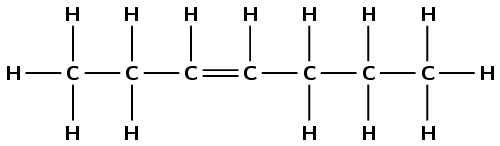
4-ethyloct-3-ene
The prefix oct- tells us there are eight carbon atoms in the longest chain containing the functional group. The suffix -3-ene tells us there is a double bond between the third and fourth carbon atoms. 4-ethyl tells us that there is an ethyl (\(\text{CH}_{3}\text{CH}_{2}-\)) branched group attached to the fourth carbon atom.
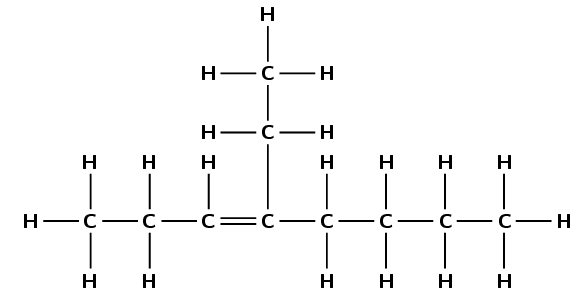
The suffix for an alkyne is -yne.
Give the IUPAC name for the following compound:
There is a triple bond between two of the carbon atoms, so this compound is an alkyne. The suffix will be -yne.
The functional group is a triple bond, so the longest chain must contain the triple bond. There are six carbon atoms in the longest chain. The prefix of the compound's name will be hex-.
In this example, you will need to number the carbons from right to left so that the triple bond is between carbon atoms with the lowest numbers (the suffix for the compound will therefore be -2-yne).
There is a methyl (\(\text{CH}_{3}\)) group attached to the fifth carbon (remember we have numbered the carbon atoms from right to left).
If we follow this order, the name of the compound is 5-methylhex-2-yne.
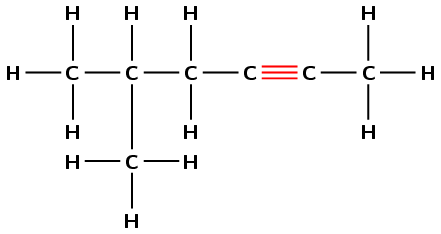
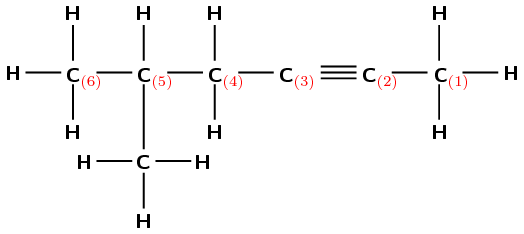
Give the IUPAC name for the following compound:
There are two triple bonds. The suffix will therefore be -diyne.
The functional group is a triple bond, so the longest chain must contain all triple bonds. The longest carbon chain contains seven carbon atoms, therefore the prefix will be hept-.

Numbering from left to right (shown in red) the first triple bond is on carbon 1 and the second is on carbon 5. The suffix will therefore be -1,5-diyne.
(Numbering from right to left (shown in blue) will give the suffix -2,6-diyne, and is incorrect).
There are no branched groups for this molecule.
The name of the compound is hept-1,5-diyne.
Draw the structural and condensed structural formula for the organic compound
6-methylhept-3-yne
The suffix -3-yne means that this compound is an alkyne and there must be a triple bond located on carbon number 3.
The prefix for the compound is hept- so there must be seven carbons in the longest chain.

There is a methyl group located on carbon number 6.
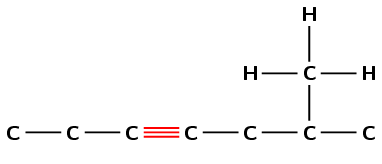
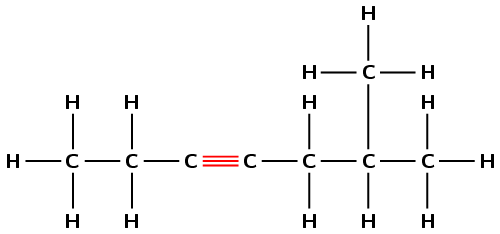
First condense the main chain: \(\text{CH}_{3}\text{CH}_{2}\text{CCCH}_{2}\text{CHCH}_{3}\)
Then add the side chains (in brackets) on the relevant carbon atoms:
\(\text{CH}_{3}\text{CH}_{2}\text{CCCH}_{2}\text{CH}(\text{CH}_{3})\text{CH}_{3}\)
Give the structural formula for each of the following alkynes:
ethyne
The prefix eth- tells us there are two carbon atoms in the longest chain. The suffix -yne tells us there is a triple bond between the carbon atoms.
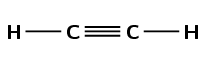
pent-1-yne
The prefix pent- tells us there are five carbon atoms in the longest chain. The suffix -1-yne tells us there is a triple bond between the first and second carbon atoms.
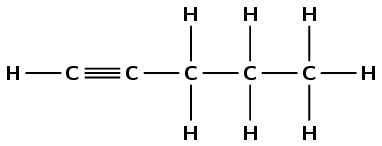
5-methylhept-3-yne
The prefix hept- tells us there are seven carbon atoms in the longest chain. The suffix -3-yne tells us there is a triple bond between the third and fourth carbon atoms. 5-methyl tells us there is a methyl branched chain on the fifth carbon atom.
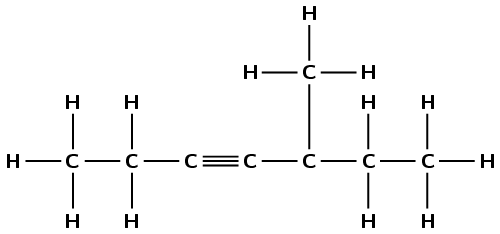
Give the IUPAC names for the following alkynes:
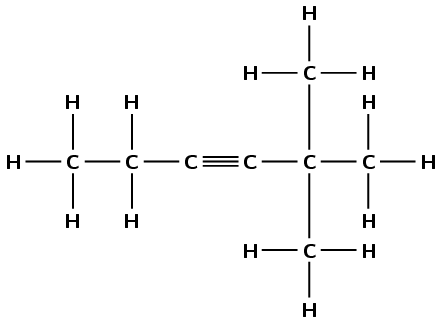
There is a triple carbon-carbon bond. This compound is an alkyne and will have the suffix -yne. There are six carbon atoms in the longest chain, therefore the prefix will be hex-. The triple bond is between the third and fourth carbon atoms regardless of how you number the chain (-3-yne). There are two branched methyl groups. Depending on the order of numbering they are either on the fourth carbon atom (left to right) or the second carbon atom (right to left). The lower numbering is correct (right to left) and as there are two methyl groups this is 2,2-dimethyl. The molecule is 2,2-dimethylhex-3-yne.
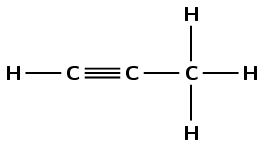
There is a triple carbon-carbon bond. This compound is an alkyne and will have the suffix -yne. There are three carbon atoms in the longest chain, therefore the prefix will be prop-. The triple bond is between the first and second carbon atoms (numbering from the left) making the suffix -1-yne. There are no branched groups. This molecule is prop-1-yne or propyne.
\(\text{CH}_{3}\text{CCCH}_{3}\)
Draw the structure:
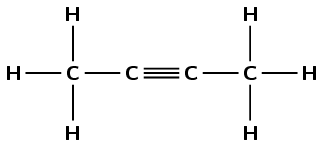
There is a triple carbon-carbon bond. This compound is an alkyne and will have the suffix -yne. There are four carbon atoms in the longest chain, therefore the prefix will be but-. The triple bond is between the second and third carbon atoms regardless of how you number the chain (-2-yne). There are no branched groups. This molecule is but-2-yne or 2-butyne.
Model kits are a great way to help learners understand and visualise organic molecules. If you do not have access to model kits however, substitutes can be used. A bag of jelly tots (or a similar soft sweet) would work just as well, using toothpicks to represent the bonds. It is also possible to make your own playdough (which can be coloured for different atoms using food colourants). A recipe is given here:
2 cups flour, 2 cups warm water, 1 cup salt, 2 tablespoons vegetable oil, 1 tablespoon of cream of tartar (this improves elasticity, and is optional).
Mix all the ingredients together over a low heat and stir continually.
When the dough pulls away from the sides of the pot, remove it from the heat and allow to cool.
If your dough is still sticky just cook it a bit longer.
Once cool, knead the dough until it becomes smooth, then separate and add food colourant, different colours for different types of atoms.
The dough is ready. Remember to store it in an airtight container between uses. If it dries out a bit, knead in a bit of water.
The full process is provided on www.instructables.com. This playdough can be used for all the model building activities.
Be careful to keep the playdough away from animals, as it is edible, but contains large amounts of salt.
An example of ethane, ethene and ethyne built with atomic model kits is given in the picture below:
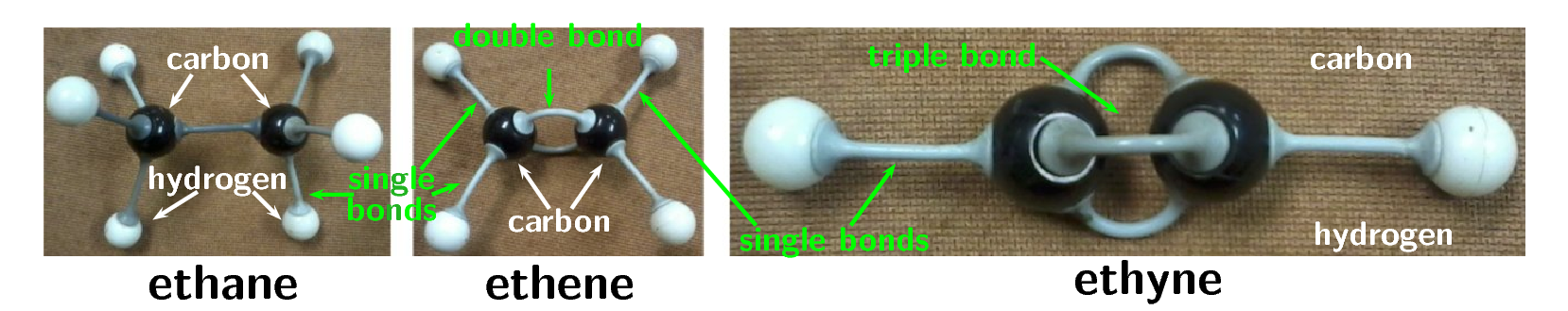
Using atomic model kits, build the molecules of methane, propane, butane, pentane and octane. If you don't have atomic model kits, jelly tots (or playdough) and toothpicks will work just as well. Use one colour jelly tot for the carbon atoms and one for the hydrogen atoms.
Build the molecules of prop-1-ene, but-1-ene, pent-1-ene and oct-1-ene. Use two toothpicks to represent a double bond. You should see that all these compounds have a similar formula. Remember, they all have the general formula \(\text{C}_{\text{n}}\text{H}_{2\text{n}}\).
Build the molecules of prop-1-yne, but-1-yne, pent-1-yne and oct-1-yne. Use three toothpicks to represent a triple bond. You should see that all these compounds have a similar formula. Remember, they are all alkynes.
Give the structural formula for each of the following compounds:
oct-2-ene
The prefix oct- tells us there are eight carbon atoms in the longest chain. The suffix -2-ene tells us there is a double bond between the second and third carbon atoms.
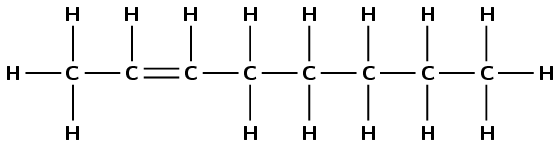
3-methylhexane
The prefix hex- tells us there are six carbon atoms in the longest chain. The suffix -ane tells us this is an alkane and that there are only single carbon-carbon bonds and no other functional groups. 3-methyl tells us there is a branched methyl group on the third carbon atom.
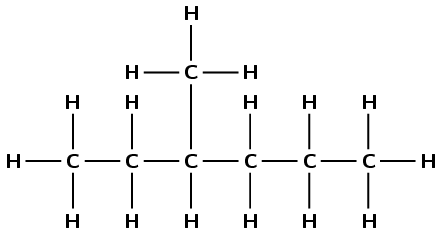
hept-3-yne
The prefix hept- tells us there are seven carbon atoms in the longest chain. The suffix -3-yne tells us that this is an alkyne and there is a triple bond between the third and fourth carbon atoms.
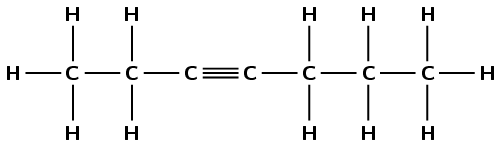
4-ethyl-4-methylhept-2-yne
The prefix hept- tells us there are seven carbon atoms in the longest chain. The suffix -2-yne tells us this is an alkyne and there is a triple bond between the second and third carbon atoms. The 4-ethyl tells us there is an ethyl (\(\text{CH}_{3}\text{CH}_{2}-\)) branched group on the fourth carbon atom. The 4-methyl tells us there is a methyl (\(\text{CH}_{3}-\)) branched group on the fourth carbon atom.
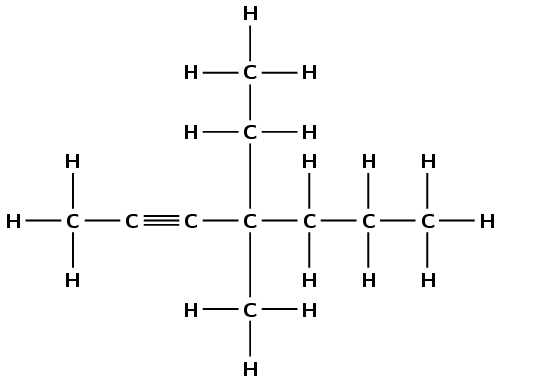
pentane
The prefix pent- tells us there are five carbon atoms in the longest chain. The suffix -ane tells us this is an alkane.
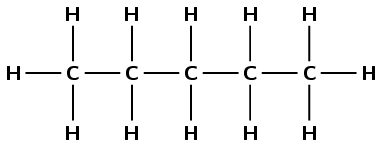
2-methylbut-1-ene
The prefix but- tells us there are four carbon atoms in the longest chain containing the functional group. The suffix -1-ene tells us that this is an alkene and there is a double bond between the first and second carbon atoms. 2-methyl tells us that there is a methyl (\(\text{CH}_{3}-\) group attached to the second carbon atom.
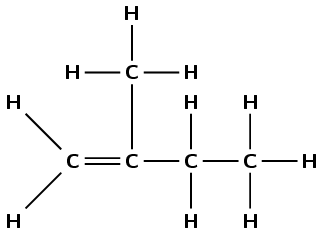
propyne
The prefix prop- tells us there are three carbon atoms in the longest chain. The suffix -yne tells us there is a triple carbon-carbon bond between the first and second carbon atoms.
Give the IUPAC name for each of the following organic compounds:
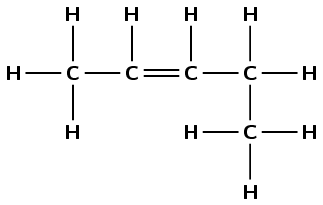
The compound has a double carbon-carbon bond and no other functional groups. Therefore it is an alkene and the suffix is -ene. There are five carbon atoms in the longest chain and so the prefix is pent-. There are no branched groups. The double bond occurs between carbons 2 and 3. The compound is 2-pentene or pent-2-ene.
Note that the way we number the carbon atoms matters. The double bond is given the lowest possible number and so this compound is not pent-3-ene.
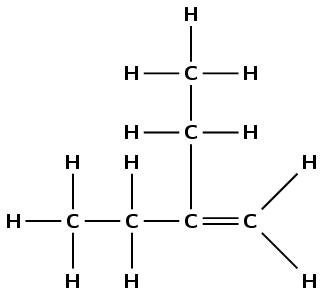
There is a double carbon-carbon bond, therefore the compound is an alkene and the suffix is -ene. There are four carbon atoms in the main chain (the longest chain containing the functional group) so the prefix is but-. There is an ethyl group on the second carbon atom. The double bond occurs between carbons 1 and 2. The compound is therefore 2-ethylbut-1-ene .
Remember that you have to have the functional group in the main chain so the compound is not a substituted pentane.
\(\text{CH}_{3}\text{CH}_{2}\text{CH}_{2}\text{CH}_{2}\text{CH}_{2}\text{CH}_{3}\)
Draw the structural representation:

There are only single carbon-carbon bonds in this compound and no other functional groups, therefore it is an alkane and the suffix is -ane. There are six carbon atoms in the longest chain so the prefix is hex-. There are no branched groups in this compound. This molecule is hexane.
\(\text{CH}_{3}\text{CH}_{2}\text{CCH}\)
Draw the structural representation of the compound:
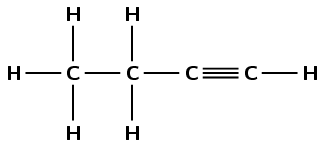
The compound has a triple carbon-carbon bond, therefore it is an alkyne and the suffix is -yne. There are four carbon atoms in the longest chain so the prefix is but-. There are no branched chains. The double bond occurs between the first and second carbon atoms. The compound is therefore but-1-yne or butyne.
Note that the way we number the carbon atoms matters. The double bond is given the lowest possible number and so this compound is not but-3-yne.
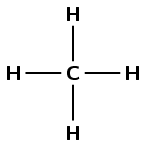
There are only single bonds in this compound and no other functional groups, therefore it is an alkane and the suffix is -ane. There is one carbon atom in the longest chain so the prefix is meth-. There are no branched groups in this compound. This molecule is methane.
\(\text{C}_{2}\text{H}_{2}\)
Draw the structural representation of the compound:
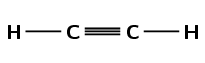
The compound has a triple carbon-carbon bond, therefore it is an alkyne and the suffix is -yne. There are two carbon atoms in the longest chain so the prefix is eth-. There are no branched chains. There are only two carbon atoms, so the triple bond has to be between them. The compound is ethyne.
\(\text{CH}_{3}\text{CHCH}_{2}\)
Draw the structural representation of the compound:
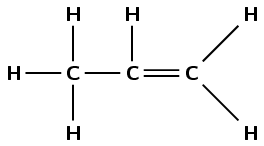
The compound has a double carbon-carbon bond, therefore it is an alkene and the suffix is -ene. There are three carbon atoms in the longest chain so the prefix is prop-. There are no branched chains. The double bond occurs between the first and second carbon atoms. The compound is propene.
Note that the way we number the carbon atoms matters. The double bond is given the lowest possible number and so this compound is not prop-2-ene. Also, we do not include the 1 in the name as this is the only position that the double bond can occur in propene.
All the same rules apply when naming the alkyl halides as for naming the hydrocarbons. We will only be dealing with the haloalkanes (i.e. there are no other functional groups). The halogen atom is treated in the same way as a branched group.
Give the IUPAC name for the following compound:
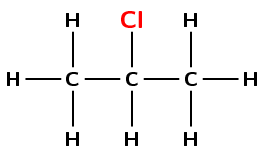
There is a halogen atom and no other functional group. This compound is therefore a haloalkane, and will have the suffix -ane.
There are three carbons in the longest chain containing the halogen atom. The prefix is prop-.
You need to number the carbon atoms so that the halogen atom is on the carbon atom with the lowest number. In this case you can number from either side.
The halogen is a chlorine atom. It is attached to carbon number 2 and so will have the name 2-chloro.
There are no branched groups in this compound.
The name of the compound is 2-chloropropane.
Give the IUPAC name for the following compound:
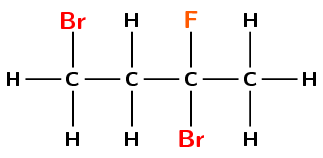
There are three halogen atoms and no other functional groups. This compound is therefore a haloalkane, and will have the suffix -ane.
There are four carbons in the longest chain containing all the halogen atoms. The prefix for this compound will be but-.
You need to number the carbon atoms so that the halogen atoms are on the carbon atoms with the lowest numbers. You must number from left to right here so that one halogen atom is on carbon 1 and two halogen atoms are on carbon 3.
There are two halogen atoms that are bromine atoms and one that is fluorine. One bromine is attached to carbon 1 and one is attached to carbon 3. The fluorine atom is attached to carbon 3. So you have 1,3-dibromo- and 3-fluoro.
There are no branched groups in this compound.
The name of the compound is 1,3-dibromo-3-fluorobutane. Note that we place the halogens in alphabetical order: bromo (ignore the di/tri/tetra) is before fluoro.
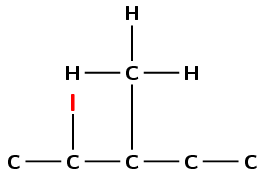
Draw the structural and condensed structural formula for the organic compound
2-iodo-3-methylpentane
This compound has the suffix -ane, but also contains a halogen atom. It is therefore a haloalkane. Note that the methyl and iodo are written in alphabetical order.
The prefix is pent- therefore there are 5 carbons in the longest chain.
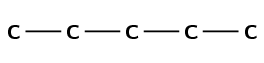
There is an iodine atom on the second carbon atom, and a methyl branched group on the third carbon atom.
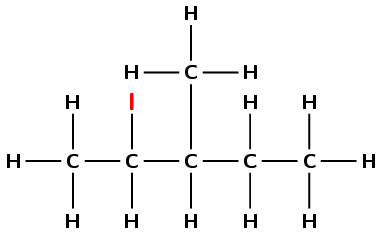
First condense the main chain: \(\text{CH}_{3}\text{CHCHCH}_{2}\text{CH}_{3}\)
Then add the side chains and halogen atoms (in brackets) on the relevant carbon atoms: \(\text{CH}_{3}\text{CH}(\text{I})\text{CH}(\text{CH}_{3})\text{CH}_{2}\text{CH}_{3}\)
Give the structural representation for the following haloalkanes:
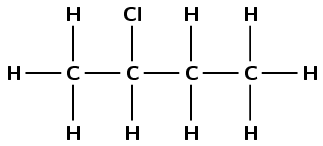
2-chlorobutane
The but- tells us that there are four carbon atoms in the longest chain. The -ane tells us there are only single carbon-carbon bonds. The 2-chloro means there is a chlorine atom attached to the second carbon atom.
1-bromopropane
The prop- tells us that there are three carbon atoms in the longest chain. The -ane tells us there are only single carbon-carbon bonds. The 1-bromo means there is a bromine atom attached to the first carbon atom.
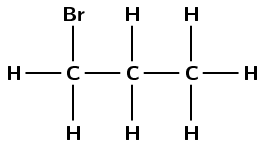
2,3-difluoropentane
The pent- tells us there are five carbon atoms in the longest chain. The -ane tells us there are only single carbon-carbon bonds. The 2,3-difluoro means there are two fluorine atoms, one attached to carbon 2 and the other attached to carbon 3.
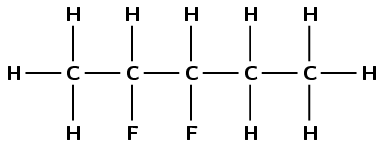
Give the IUPAC name for the following haloalkanes:
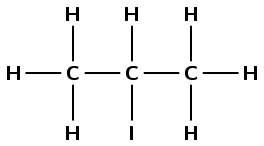
There is a halogen atom and only single carbon-carbon bonds, therefore this is a haloalkane and the suffix is -ane. There are three carbon atoms in the longest chain, therefore the prefix is prop-. There is an iodine atom attached to the second carbon atom (2-iodo). There are no branched groups. Therefore this molecule is 2-iodopropane.
\(\text{CH}_{3}\text{CH}(\text{Br})\text{CH}(\text{CH}_{3})\text{CH}_{2}\text{CH}_{2}\text{CH}_{3}\)
Draw the structural representation:
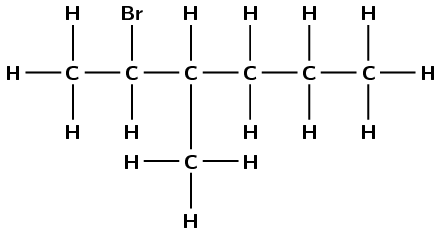
There is a halogen atom and only single carbon-carbon bonds, therefore this is a haloalkane and the suffix is -ane. There are six carbon atoms in the longest chain, therefore the prefix is hex-. There is a bromine atom attached to the second carbon atom (2-bromo). There is a branched methyl group attached to the third carbon atom (3-methyl). This molecule is
2-bromo-3-methylhexane.
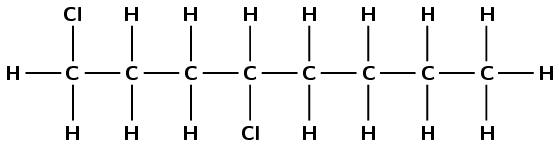
There are halogen atoms and only single carbon-carbon bonds, therefore this is a haloalkane and the suffix is -ane. There are eight carbon atoms in the longest chain, therefore the prefix is oct-. There are two chlorine atoms, one attached to the first carbon atom and one attached to the fourth carbon atom (1,4-dichloro). There are no branched groups. This molecule is 1,4-dichlorooctane.
\(\text{CH}_{2}(\text{F})\text{C}(\text{I})_{2}\text{CH}_{2}\text{CH}_{3}\)
Draw the structural representation:
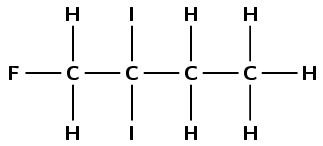
There are halogen atoms and only single carbon-carbon bonds, therefore this is a haloalkane and the suffix is -ane. There are four carbon atoms in the longest chain containing all the halogen atoms, therefore the prefix is but-. There is a fluorine atom attached to the first carbon atom (1-fluoro). There are two iodine atoms attached to the second carbon atom (2,2-diiodo). These must be put in alphabetical order, ignoring any prefixes on the halogen atoms. F comes before i (1-fluoro-2,2-diiodo). This molecule is 1-fluoro-2,2-diiodobutane
The rules used to name the alcohols are similar to those already discussed for the hydrocarbons. The suffix of an alcohol is –ol (see Table 4.5).
Give the IUPAC name for the following organic compound
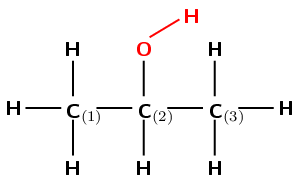
The compound has an \(-\text{OH}\) (hydroxyl) functional group and is therefore an alcohol. The compound will have the suffix -ol.
There are three carbon atoms in the longest chain that contains the functional group. The prefix for this compound will be prop-. As there are only single bonds between the carbon atoms, the prefix includes an to become propan-.
In this case, it doesn't matter whether you start numbering from the left or right. The hydroxyl group will still be attached to second carbon atom (-2-ol).
There are no branched groups in this compound.
The compound's name is propan-2-ol or 2-propanol.
Give the IUPAC name for the following compound:
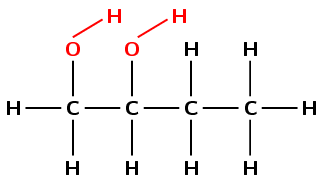
The compound has an \(-\text{OH}\) (hydroxyl) functional group and is therefore an alcohol. There are two hydroxyl groups in the compound, so the suffix will be -diol.
There are four carbon atoms in the longest chain that contains the functional group (but-) and only single bonds (an-). The prefix for this compound will be butan-.
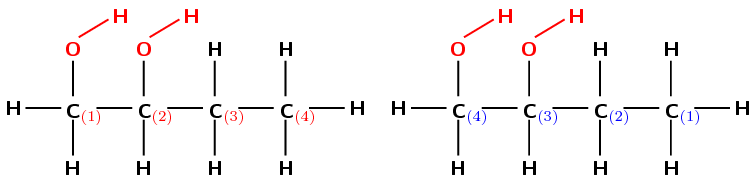
There are two hydroxyl groups attached to the main chain. If we number as shown in red (on the left) they are attached to the first and second carbon atoms. If we number as shown in blue (on the right) they are attached to the third and fourth carbon atoms.
The functional groups should have the lowest numbers possible. Therefore the red numbering is correct. The hydroxyl groups are attached to the first and second carbon atoms (1,2-diol).
There are no branched groups in this compound.
The compound's name is butan-1,2-diol.
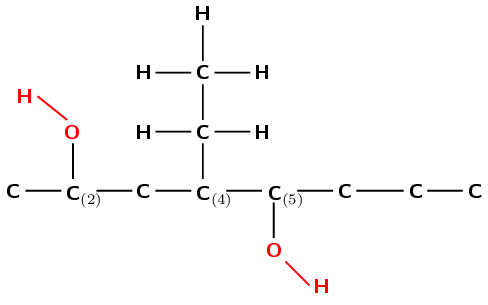
Draw the structural and condensed structural representations for the organic compound 4-ethyloctan-2,5-diol
The compound has the suffix -ol. It is therefore an alcohol.
The prefix is oct- therefore there are 8 carbons in the longest chain containing the functional group.
There is one \(-\text{OH}\) attached to carbon 2 and one attached to carbon 5. There is also an ethyl (\(-\text{CH}_{2}\text{CH}_{3}\)) branched group attached to carbon 4.
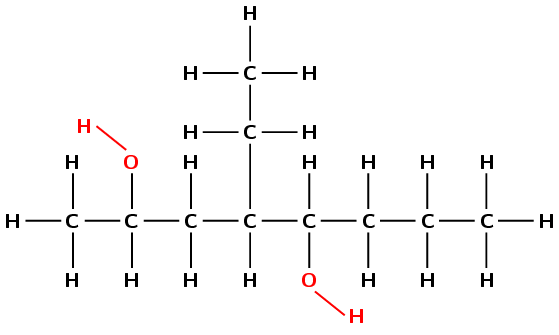
First condense the main chain: \(\text{CH}_{3}\text{CHCH}_{2}\text{CHCHCH}_{2}\text{CH}_{2}\text{CH}_{3}\)
Then add the side chains and alcohol functional groups (in brackets) on the relevant carbon atoms: \(\text{CH}_{3}\text{CH}(\text{OH})\text{CH}_{2}\text{CH}(\text{CH}_{2}\text{CH}_{3})\text{CH}(\text{OH})\text{CH}_{2}\text{CH}_{2}\text{CH}_{3}\)
The structural representation of butan-1-ol built using an atomic model kit is given below:
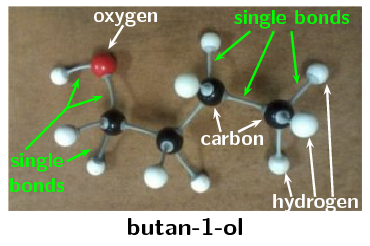
Using atomic model kits, build the molecules of methanol, ethanol, propan-1-ol, pentan-1-ol and octan-1-ol. If you don't have an atomic model kit remember that you can use jelly tots (or playdough) and toothpicks. Use different colour jelly tots to represent the different atoms.
You should see that all these compounds have a similar formula. Remember, they belong to the homologous series of the alcohols. What is the general formula for this series?
Try placing the hydroxyl group at different positions within the molecule. Does this make any difference to the total number of carbon, hydrogen and oxygen atoms in the molecule?
Give the structural representation of each of the following organic compounds:
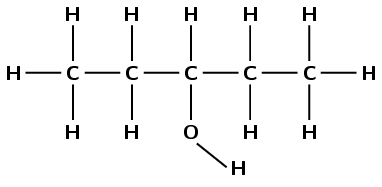
pentan-3-ol
The prefix pentan- tells us there are five carbon atoms in the longest chain and only single carbon-carbon bonds. The suffix -3-ol tells us there is a hydroxyl (\(-\text{OH}\)) group on the third carbon atom.
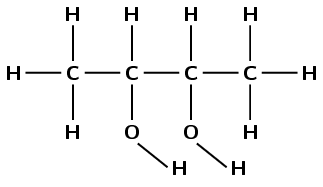
butan-2,3-diol
The prefix butan- tells us there are four carbon atoms in the longest chain and only single carbon-carbon bonds. The suffix -2,3-diol tells us there are two hydroxyl groups, one attached to the second carbon atom and one attached to the third carbon atom.
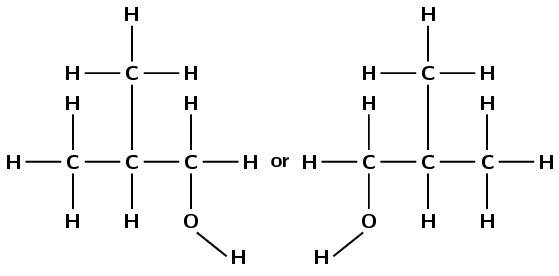
2-methylpropan-1-ol
The prefix propan- tells us there are three carbon atoms in the longest chain and only single carbon-carbon bonds. The suffix -1-ol tells us there is a hydroxyl group attached to the first carbon atom. 2-methyl tells us there is a methyl branched group attached to the second carbon atom.
Give the IUPAC name for each of the following:
\(\text{CH}_{3}\text{CH}_{2}\text{CH}(\text{OH})\text{CH}_{3}\)
Draw the structural representation of the compound:
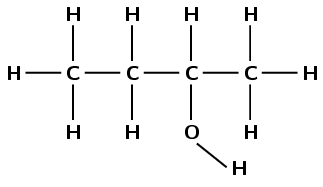
There is a hydroxyl group, therefore the compound is an alcohol and the suffix is -ol. There are four carbon atoms in the longest chain so the prefix is but-. There are only single carbon-carbon bonds, therefore the prefix becomes butan-. There are no branched groups. The hydroxyl group is attached to the second carbon atom. The molecule is 2-butanol or butan-2-ol.
Note that the way we number the carbon atoms matters. The hydroxyl group is given the lowest possible number and so this compound is not butan-3-ol.
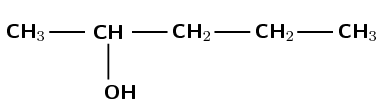
There is a hydroxyl group, therefore the compound is an alcohol and the suffix is -ol. There are five carbon atoms in the longest chain so the prefix is pent-. There are only single carbon-carbon bonds, therefore the prefix becomes pentan-. There are no branched groups. The hydroxyl group is attached to the second carbon atom. The molecule is 2-pentanol or pentan-2-ol.
Note that the way we number the carbon atoms matters. The hydroxyl group is given the lowest possible number and so this compound is not pentan-4-ol.
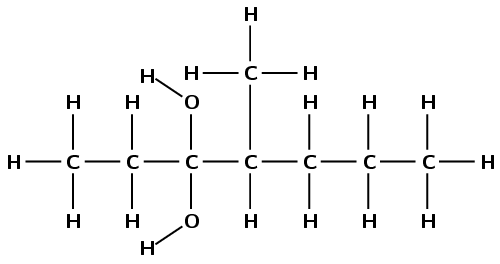
There are two hydroxyl groups, therefore the compound is an alcohol and the suffix is -diol. There are seven carbon atoms in the longest chain so the prefix is hept-. There are only single carbon-carbon bonds, therefore the prefix becomes heptan-. The hydroxyl groups are both attached to the third carbon atom (-3,3-diol). The branched methyl chain is attached to the fourth carbon atom. The molecule is 4-methylheptan-3,3-diol.
Note that the way we number the carbon atoms matters. The hydroxyl groups are given the lowest possible numbers and so this compound is not 4-methylheptan-5,5-diol.
A carbonyl group consists of a carbon atom that is bonded to an oxygen atom through a double bond (C\(=\)O). There are many different functional groups that contain a carbonyl group.
If the carbonyl group is on the end of the carbon chain, the organic compound is called an aldehyde. An aldehyde has the suffix -al.
Give the IUPAC name and molecular formula for the following organic compound
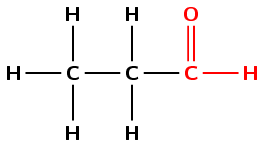
The compound has a C\(=\)O (carbonyl) group and no other functional groups. It is therefore either an aldehyde or a ketone. The carbonyl group is on the last (terminal) carbon in the main chain so the compound is an aldehyde. It will have the suffix -al.
There are three carbons in the longest chain that contains the functional group. The prefix for this compound will be prop-. As there are only single bonds between the carbon atoms, the prefix becomes propan-.
The carbon atoms will be numbered so that the carbon atom of the aldehyde group has the lowest number possible. In this case that is from right to left.
There are no branched groups in this compound.
The compound's name is propanal (there is no need to say propan-1-al as by definition all aldehydes are -1-al).
There are 3 carbon atoms, 6 hydrogen atoms and 1 oxygen atom so the molecular formula is \(\text{C}_{3}\text{H}_{6}\text{O}\).
(Remember that there is no structural information given by the molecular formula)
Ketone is pronounced keytone. Therefore propanone is pronounced propanown.
If the carbonyl group is in the middle of the carbon chain, the compound is called a ketone. A ketone has the suffix -one.
Note that butanone can only be butan-2-one. If the carbonyl group were on carbon 1 it would be an aldehyde, while if it were on carbon 3 we would simply count from the other side of the molecule. The 2 is still required for IUPAC naming however.
Give the IUPAC name and molecular formula for the following compound:
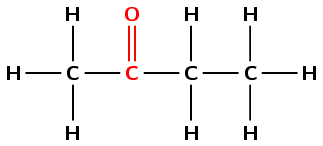
The compound has a C\(=\)O (carbonyl) group and no other functional groups. It is therefore either an aldehyde or a ketone. The carbonyl group is not at the end of the chain. Therefore the compound is a ketone and the suffix will be -one.
There are four carbons in the longest chain that contains the functional group, and only single carbon-carbon bonds. The prefix for this compound will be butan-.
The carbon atoms will be numbered from left to right so that the carbon atom of the ketone group has the lowest number possible (-2-one).
There are no branched groups in this compound.
The compound's name is butan-2-one or 2-butanone.
There are 4 carbon atoms, 8 hydrogen atoms and 1 oxygen atom so the molecular formula is \(\text{C}_{4}\text{H}_{8}\text{O}\).
(Remember that there is no structural information given by the molecular formula)
Give the IUPAC name for the following compound:
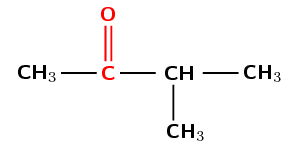
The compound has a C\(=\)O (carbonyl) group and no other functional groups. It is therefore either an aldehyde or a ketone. The carbonyl group is not at the end of the chain. Therefore the compound is a ketone and the suffix will be -one.
The longest carbon chain that contains the functional group has four carbon atoms in it, and only single bonds. The prefix for this compound will be butan-.
The carbon atoms will be numbered from left to right so that the carbon atom of the ketone functional group has the lowest possible number. The suffix will be -2-one.
There is a branched group on carbon 3. This group has only one carbon atom. The branched group is attached to the third carbon atom (3-methyl).
The compound's name is 3-methylbutan-2-one.
Draw the structural and condensed structural representations for the organic compound 3-methylpentanal.
The compound has the suffix -al. It is therefore an aldehyde and has a C\(=\)O (carbonyl) group on the first carbon atom.
The prefix is pent- so there are 5 carbon atoms in the longest chain.
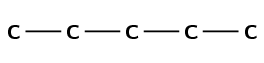
There is a C\(=\)O (carbonyl) group at the first carbon atom and a methyl group attached to the third carbon atom (3-methyl).


First condense the main chain including the carbonyl group oxygen atom: \(\text{CHOCH}_{2}\text{CHCH}_{2}\text{CH}_{3}\)
Then add the side chains on the relevant carbon atoms: \(\text{CHOCH}_{2}\text{CH}(\text{CH}_{3})\text{CH}_{2}\text{CH}_{3}\)
Draw the structural and condensed structural representations for the organic compound 3-methylpentan-2-one.
The compound has the suffix -one. It is therefore a ketone and has a C\(=\)O (carbonyl) group. This group cannot be on the first carbon atom.
The prefix is pent- therefore there are 5 carbon atoms in the longest chain.
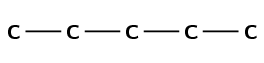
There is a C\(=\)O (carbonyl) group at the second carbon atom (-2-one) and a methyl group attached to the third carbon atom (3-methyl).
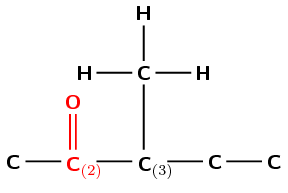
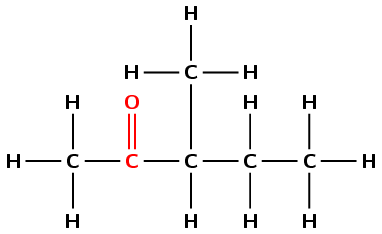
First condense the main chain including the carbonyl group oxygen atom: \(\text{CH}_{3}\text{COCHCH}_{2}\text{CH}_{3}\)
Then add the side chains on the relevant carbon atoms: \(\text{CH}_{3}\text{COCH}(\text{CH}_{3})\text{CH}_{2}\text{CH}_{3}\)
Give the IUPAC name for each of the following compounds:
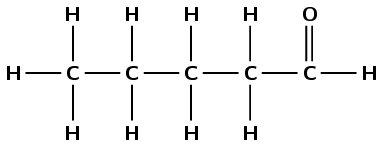
The compound has a carbonyl group and no other functional groups. Therefore it is either an aldehyde or a ketone. The carbonyl group carbon atom is the last (terminal) carbon atom. Therefore this is an aldehyde and the suffix is -al. There are five carbon atoms in the longest chain, therefore the prefix is pentan-. This molecule is pentanal.
Note that no number is needed for the position of the aldehyde. We must number the chain so that the carbonyl group is always the first carbon atom.
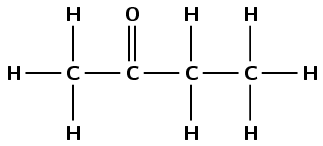
The compound has a carbonyl group and no other functional groups. Therefore it is either an aldehyde or a ketone. The carbonyl group carbon atom is not the last carbon atom. Therefore this is a ketone and the suffix is -one. There are four carbon atoms in the longest chain, therefore the prefix is butan-. This molecule is butan-2-one or 2-butanone.
Note that the way we number the carbon atoms is important. This compound is not butan-3-one.
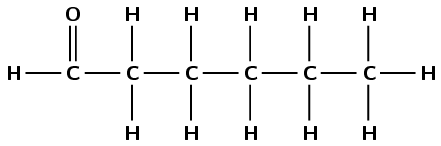
The compound has a carbonyl group and no other functional groups. Therefore it is either an aldehyde or a ketone. The carbonyl group carbon atom is a terminal carbon atom. Therefore this is an aldehyde and the suffix is -al. There are six carbon atoms in the longest chain, therefore the prefix is hexan-. This molecule is hexanal.
Note that no number is needed for the position of the aldehyde. We must number the chain so that the carbonyl group is always the first carbon atom.
\(\text{HCHO}\)
Draw the structural representation:
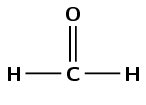
The compound has a carbonyl group and no other functional groups. Therefore it is either an aldehyde or a ketone. The carbonyl group carbon atom is at the end of the chain. Therefore this is an aldehyde and the suffix is -al. There is one carbon atom in the longest chain, therefore the prefix is methan-. This molecule is methanal.
\(\text{CH}_{3}\text{CH}_{2}\text{COCH}_{2}\text{CH}_{3}\)
Draw the structural representation:
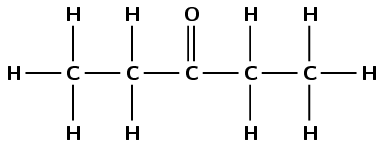
The compound has a carbonyl group and no other functional groups. Therefore it is either an aldehyde or a ketone. The carbonyl group carbon atom is the third carbon atom. Therefore this is a ketone and the suffix is -3-one. There are five carbon atoms in the longest chain, therefore the prefix is pentan-. This molecule is pentan-3-one or 3-pentanone.
Give the structural representation for the following:
ethanal
The prefix eth- tells us that there are two carbon atoms in the longest chain. The suffix -al tells us that the first (or last) carbon atom is part of a carbonyl group.
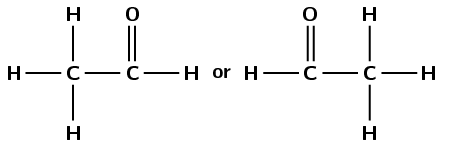
propanone
The prefix prop- tells us that there are three carbon atoms in the longest carbon chain. The suffix -one tells us that there is a carbonyl group and it is not on the end of the chain. There is only one carbon atom not on the end: the second carbon atom.
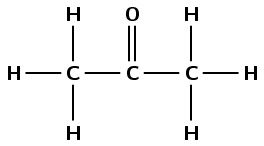
heptan-3-one
The prefix hept- tells us there are seven carbon atoms in the longest chain. The suffix -3-one tells us that the third carbon atom is part of a carbonyl group.
Carboxylic acids are characterised by having a carboxyl group, which has the formula \(-\text{COOH}\). In a carboxyl group a carbon atom is double-bonded to an oxygen atom (a carbonyl group), and is also bonded to a hydroxyl (alcohol) group. The IUPAC suffix for a carboxylic acid is -oic acid.
Give the IUPAC name and molecular formula for the following compound:
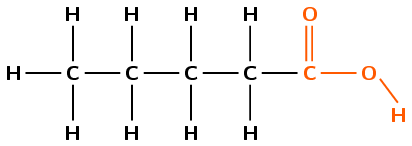
The compound has a \(-\text{COOH}\) group and is therefore a carboxylic acid. The suffix will be -oic acid.
There are five carbon atoms in the longest chain that contains the functional group, and only single bonds between carbon atoms. The prefix for this compound is pentan-.
The carbon atoms will be numbered from right to left so that the carboxylic acid functional group has the lowest numbered carbon atom.
There are no branched groups in this compound.
The compound's name is pentanoic acid.
There are 5 carbon atoms, 10 hydrogen atoms and 2 oxygen atoms so the molecular formula is \(\text{C}_{5}\text{H}_{10}\text{O}_{2}\).
Draw the structural and condensed structural representations for the organic compound 2-ethylhexanoic acid.
The compound has the suffix -oic acid. It is therefore a carboxylic acid and has a \(-\text{COOH}\) group. This group can only be on carbon 1 (at the end of the carbon chain).
The prefix is hex- therefore there are 6 carbons in the longest chain.
The \(-\text{COOH}\) group contains the first carbon atom. The ethyl group is attached to the second carbon atom.
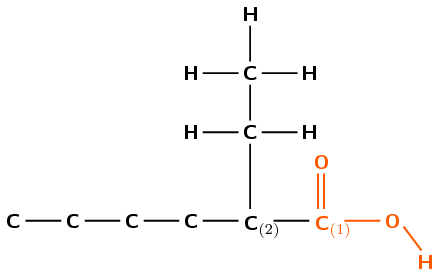
Remember that the main carbon chain must contain the functional group. This is hexanoic acid and not a substituted heptane.
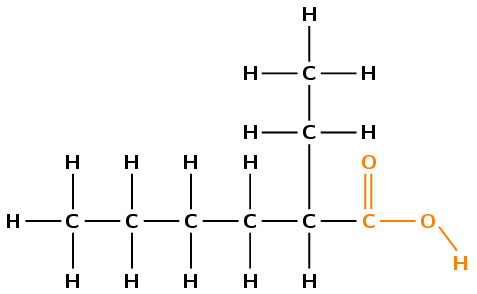
First condense the main chain, including the \(-\text{COOH}\) functional group:
\(\text{CH}_{3}\text{CH}_{2}\text{CH}_{2}\text{CH}_{2}\text{CHCOOH}\)
Then add the side chains (in brackets) on the relevant carbon atoms:
\(\text{CH}_{3}\text{CH}_{2}\text{CH}_{2}\text{CH}_{2}\text{CH}(\text{CH}_{2}\text{CH}_{3})\text{COOH}\)
Using atomic model kits build the molecules of methanoic acid, ethanoic acid, butanoic acid, pentanoic acid and octanoic acid. If you don't have an atomic model kit remember that you can use jelly tots (or playdough) and toothpicks. Use different colour jelly tots to represent the different atoms and two toothpicks to represent double bonds.
You should see that all these compounds have a similar formula. Remember, they belong to the carboxylic acid homologous series. What is the general formula for this series?
Remember that carbon must have four bonds, oxygen must have two bonds and hydrogen can only have one bond. Thinking about this fact, is it possible to have the carboxylic acid (\(-\text{COOH}\)) group in a position other than the last (or first) carbon atom?
Give the structural representation for the following:
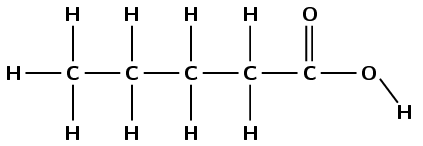
pentanoic acid
The prefix pent- tells us that there are five carbon atoms in the longest chain. The suffix -oic acid tells us that this is a carboxylic acid (\(-\)COOH).
4-ethyl-7-methyloctanoic acid
The prefix oct- tells us that there are eight carbon atoms in the longest chain. The suffix -oic acid tells us that this is a carboxylic acid (\(-\)COOH). 4-ethyl means that there is an ethyl branched group (\(\text{CH}_{3}\text{CH}_{2}-\)) attached to the fourth carbon atom. 7-methyl means that there is a methyl branched group (\(\text{CH}_{3}-\)) attached to the seventh carbon atom. Remember to always number from the carboxylic acid side of the compound.
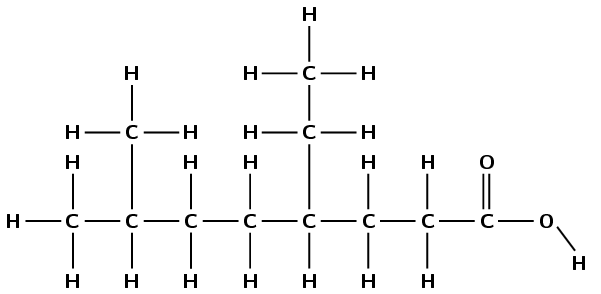
4,4-diethylheptanoic acid
The prefix hept- tells us that there are seven carbon atoms in the longest chain. The suffix -oic acid tells us that this is a carboxylic acid (\(-\)COOH). 4,4-diethyl means that there are two ethyl branched groups (\(\text{CH}_{3}\text{CH}_{2}-\)) attached to the fourth carbon atom. Remember to always number from the carboxylic acid side of the compound.
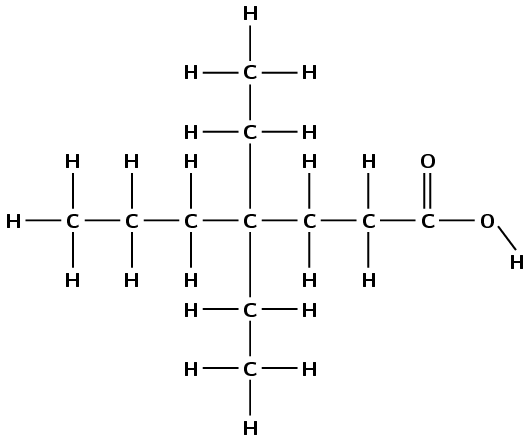
Give the IUPAC name for each of the following:
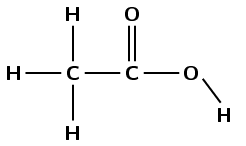
This compound contains a carbonyl group and a hydroxyl group, both attached to the same carbon atom. Therefore this is a carboxylic acid and the suffix is -oic acid. There are two carbon atoms in the longest chain, therefore the prefix is ethan-. There are no branched groups. This molecule is therefore ethanoic acid.
\(\text{CH}_{3}\text{C}(\text{CH}_{3})_{2}\text{CH}_{2}\text{CH}_{2}\text{COOH}\)
Draw the structural representation:
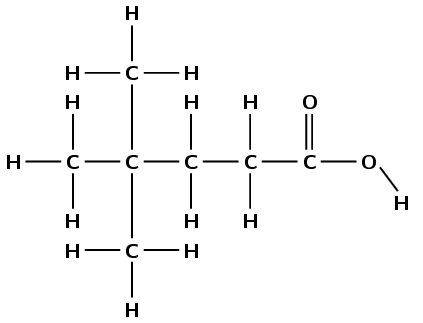
This compound contains a carbonyl group and a hydroxyl group, both attached to the same carbon atom. Therefore this is a carboxylic acid and the suffix is -oic acid. There are five carbon atoms in the longest chain, therefore the prefix is pentan-. There are two branched groups attached to the fourth carbon atom (remember to count from the carboxylic acid functional group). This molecule is therefore 4,4-dimethylpentanoic acid.
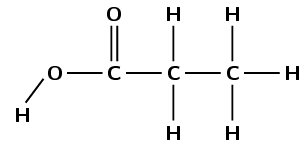
This compound contains a carbonyl group and a hydroxyl group, both attached to the same carbon atom. Therefore this is a carboxylic acid and the suffix is -oic acid. There are three carbon atoms in the longest chain, therefore the prefix is propan-. There are no branched groups. This molecule is therefore propanoic acid.
\(\text{CH}_{3}\text{CH}_{2}\text{CH}_{2}\text{CH}(\text{CH}_{2}\text{CH}_{3})\text{CH}_{2}\text{CH}(\text{CH}_{2}\text{CH}_{3})\text{COOH}\)
Draw the structural representation:
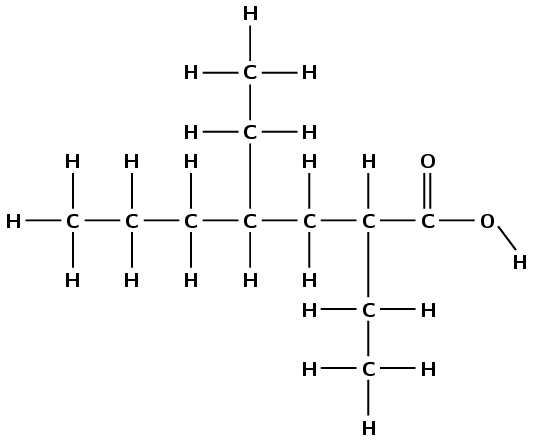
This compound has a carbonyl group and a hydroxyl group, both attached to the same carbon atom. Therefore this is a carboxylic acid and the suffix is -oic acid. There are seven carbon atoms in the longest chain, therefore the prefix is heptan-. There are two branched ethyl groups (\(\text{CH}_{3}\text{CH}_{2}-\)), one attached to the second carbon atom and one attached to the fourth carbon atom. This molecule is therefore 2,4-diethylheptanoic acid.
When an alcohol reacts with a carboxylic acid an ester is formed. A new bond is formed between the oxygen atom of the hydroxyl group and the carbonyl carbon atom of the carboxylic acid. The suffix for an ester is -oate.
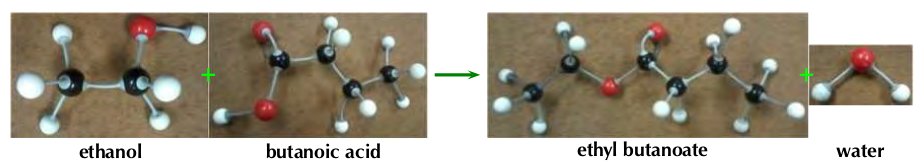
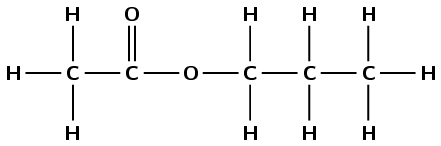
Figure 4.49: The esterification process of ethanol and butanoic acid to ethyl butanoate and water.
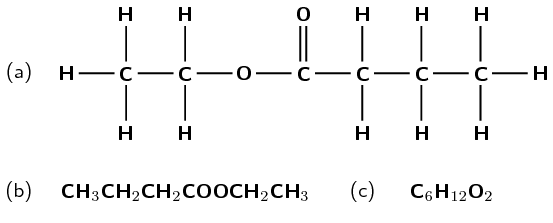
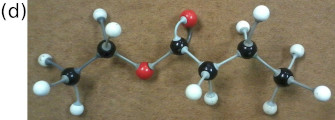
Figure 4.50: The (a) structural, (b) condensed structural and (c) molecular formula representations of ethyl butanoate. (d) An atomic model of ethyl butanoate.
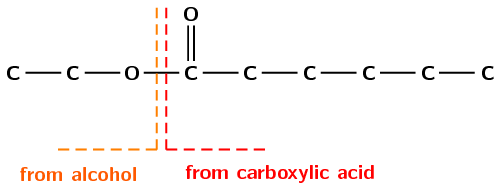
Although the part of the ester from the alcohol (ethyl) is on the right, and the part from the carboxylic acid (butan-) is on the left in Figure 4.51, when naming the structure the part of the name from the alcohol is written first (ethyl butanoate). Don't forget to count the carbon atom in the carbonyl group when determining the number of carbon atoms in the chain.
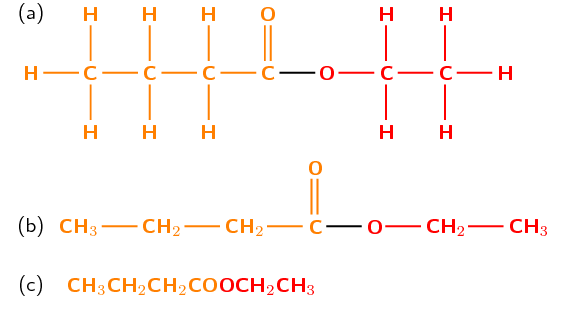
Figure 4.51: The (a) structural, (b) semi-structural structural and (c) condensed structural representations of \(\color{red}{\textbf{ethyl}}\color{orange}{\textbf{ butanoate}}\).
Give the IUPAC name for the following compound:
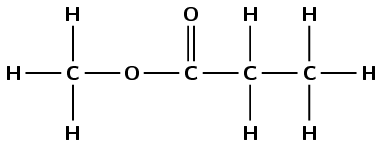
There is a \(-\)C\(=\)O (carbonyl) group as well as an oxygen atom bonded to the carbon atom of the carbonyl and another carbon atom. This is therefore an ester and the suffix is -oate.
An ester is a carboxylic acid derivative. Divide the molecule in two with the carbonyl group on one side and the oxygen bonded to two carbon atoms on the other.
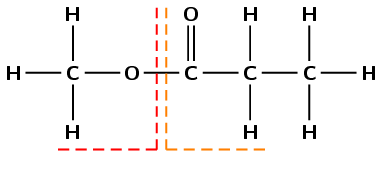
The part containing the oxygen atom bonded to two different carbon atoms was formed from the alcohol and is on the left here. The part containing the carbonyl group was formed from the carboxylic acid and is on the right here.
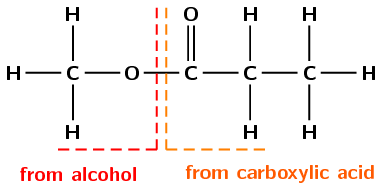
There is only one carbon atom in the left-hand chain (from the alcohol). Therefore this will be methyl. There are three carbon atoms in the right-hand chain (from the carboxylic acid) therefore the prefix will be propan-.
The compound's name is methyl propanoate.
Draw the structural and condensed structural representations for the organic compound ethyl hexanoate.
The compound has the suffix -oate. It is therefore an ester and has a \(-\)C\(=\)O (carbonyl) group as well as an oxygen atom bonded to the carbon atom of the carbonyl and another carbon atom.
The ethyl tells us that there are two carbon atoms in the part of the chain from the alcohol. The prefix hex- tells us that there are six carbon atoms from the part of the chain from the carboxylic acid.

The oxygen atom bonded to two different carbon atoms is located between the two sections. The \(-\)C\(=\)O (carbonyl) group is located at the first carbon atom of the carboxylic acid chain.
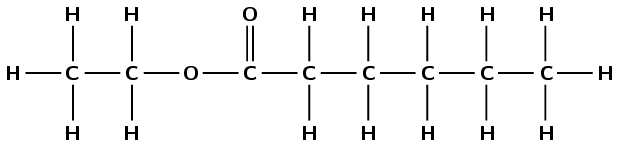
Condense the part of the compound that came from the carboxylic acid first, so start from the right here: \(\text{CH}_{3}\text{CH}_{2}\text{CH}_{2}\text{CH}_{2}\text{CH}_{2}\text{COO}\)
The first O is for the \(-\)C\(=\)O, the second is from the \(-\text{O}-\). Now condense the part that came from the alcohol, starting from the \(-\text{O}-\):
\(\text{CH}_{3}\text{CH}_{2}\text{CH}_{2}\text{CH}_{2}\text{CH}_{2}\text{COOCH}_{2}\text{CH}_{3}\)
Give the IUPAC name for each of the following compounds:
\(\text{CH}_{3}\text{COOCH}_{2}\text{CH}_{3}\)
Draw the structural representation:
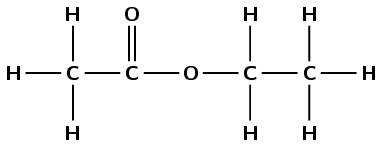
This compound contains a carbonyl group and an oxygen atom attached to two different carbon atoms. Therefore this is an ester and the suffix is -oate. There are two carbon atoms in the chain without the carbonyl group (from the alcohol), therefore this group is ethyl. There are two carbon atoms in the chain with the carbonyl group (from the carboxylic acid), therefore the prefix is ethan-. There are no branched groups. This molecule is therefore ethyl ethanoate.
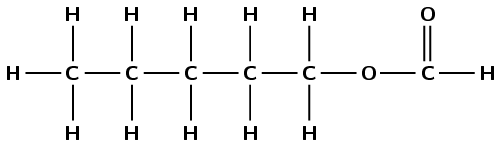
This compound contains a carbonyl group and an oxygen atom attached to two different carbon atoms. Therefore this is an ester and the suffix is -oate. There are five carbon atoms in the chain without the carbonyl group (from the alcohol), therefore this group is pentyl. There is one carbon atom in the chain with the carbonyl group (from the carboxylic acid), therefore the prefix is methan-. There are no branched groups. This molecule is therefore pentyl methanoate.
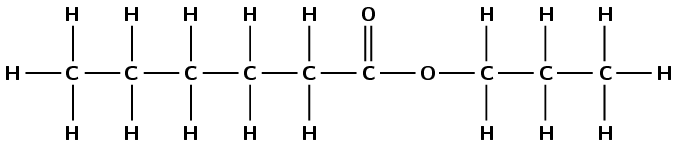
This compound contains a carbonyl group and an oxygen atom attached to two different carbon atoms. Therefore this is an ester and the suffix is -oate. There are three carbon atoms in the chain without the carbonyl group (from the alcohol), therefore this group is propyl. There are six carbon atoms in the chain with the carbonyl group (from the carboxylic acid), therefore the prefix is hexan-. There are no branched groups. This molecule is propyl hexanoate.
Give the structural representations for the following esters:
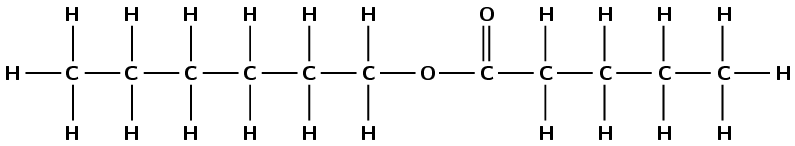
heptyl propanoate
The suffix -oate tells us that this is an ester. The heptyl tells us that there are seven carbon atoms in the chain without the carbonyl group (from the alcohol). The prefix prop- tells us that there are three carbon atoms in the chain with the carbonyl group (from the carboyxlic acid).
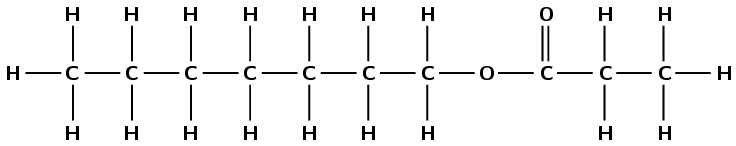
methyl octanoate
The suffix -oate tells us that this is an ester. The methyl tells us that there is one carbon atom in the chain without the carbonyl group (from the alcohol). The prefix oct- tells us that there are eight carbon atoms in the chain with the carbonyl group (from the carboyxlic acid).
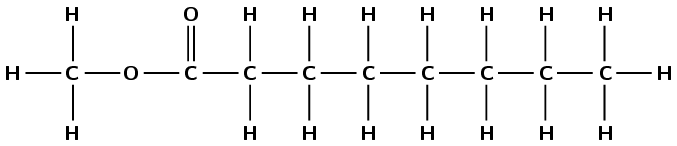
hexyl pentanoate
The suffix -oate tells us that this is an ester. The hexyl tells us that there are six carbon atoms in the chain without the carbonyl group (from the alcohol). The prefix pent- tells us that there are five carbon atoms in the chain with the carbonyl group (from the carboxylic acid).
Give the IUPAC name for the following compound:
\(\text{CH}_{3}\text{CH}_{2}\text{CH}(\text{CH}_{3})\text{CH}_{2}\text{CH}_{2}\)\(\color{red}{\textbf{COOH}}\)
(Remember that the side groups are shown in brackets after the carbon atom to which they are attached)
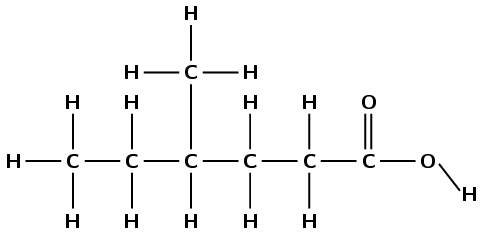
The compound has a \(-\text{COOH}\) functional group. It is therefore a carboxylic acid and the suffix is -oic acid.
There are six carbon atoms in the longest chain containing the functional group. The prefix for this compound is hexan-.
The carbon atoms should be numbered from right to left so that the carboxylic acid functional group has the lowest numbered carbon atom.
There is a branched group attached to the fourth carbon atom. This group has only one carbon atom and is therefore a methyl group (4-methyl).
The compound's name is 4-methylhexanoic acid.
Give the IUPAC name for the following compound:
\(\text{CH}_{3}\)\(\color{red}{\text{COO}}\)\(\text{CH}_{2}\text{CH}_{2}\text{CH}_{2}\text{CH}_{3}\)
The compound has a \(-\text{COO}\) functional group. It is therefore an ester and the suffix will be -oate. This can also be shown as:
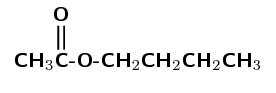
The left half of the compound contains the carbonyl group and is therefore from the carboxylic acid. The right half of the compound must be from the alcohol.
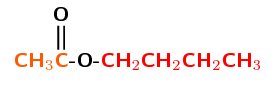
There are 2 carbon atoms in the part that contains the C\(=\)O group (from the carboyxlic acid), therefore the prefix is ethan-. There are 4 carbon atoms in part of the chain without the carbonyl group (from the alcohol), which is therefore butyl.
There are no branched groups.
The compound's name is butyl ethanoate.
Give the structural representation for the following compounds:.
3-methylpentanal
The suffix -al tells us that the compound is an aldehyde. The prefix pent- tells us that there are five carbon atoms in the longest chain. 3-methyl tells us that there is a methyl branched group on the third carbon atom.
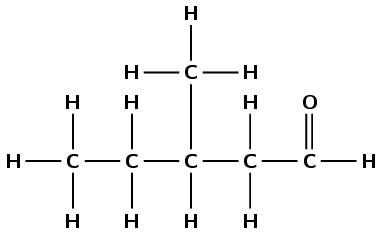
butyl pentanoate
The suffix -oate tells us that the compound is an ester. The prefix pent- tells us that there are five carbon atoms in the chain containing the carbonyl group. Butyl tells us that there are four carbon atoms in the chain that does not contain the carbonyl group. There are no branched groups.
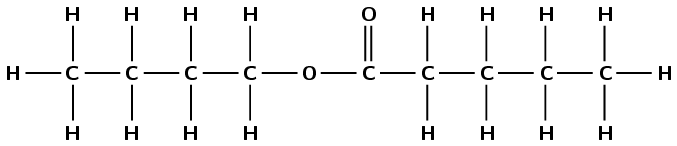
2-methylbutanoic acid
The suffix -oic acid tells us that the compound is a carboxylic acid. The prefix but- tells us that there are four carbon atoms in the longest chain. 2-methyl tells us that there is a branched methyl chain attached to the second carbon atom (remember to count from the carboxylic acid carbon atom).
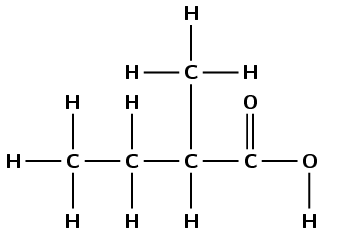
octan-4-one
The suffix -4-one tells us that the compound is a ketone and that the fourth carbon atom is the carbonyl carbon atom. The prefix oct- tells us that there are eight carbon atoms in the longest chain. There are no branched chains.
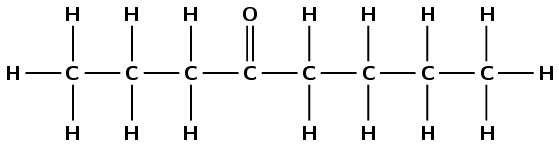
Give the IUPAC name for each of the following compounds:
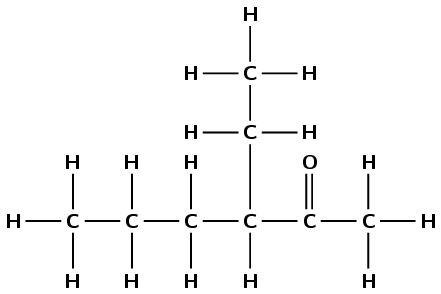
There is a carbonyl group and it is not on the first or last carbon atom. This compound must be a ketone and have the suffix -one. There are six carbon atoms in the longest chain containing the carbonyl functional group, therefore the prefix is hexan-. The carbonyl is on the second carbon atom. There is a branched ethyl group attached to the third carbon atom. This molecule is therefore 3-ethylhexan-2-one.
Note that the way you number this compound is important. This is not 4-ethylhexan-5-one.
\(\text{CH}_{3}\text{CH}_{2}\text{CH}_{2}\text{CHO}\)
Draw the structural representation:
There is a carbonyl group and it is on the first/last carbon atom. This compound must be an aldehyde and have the suffix -al. There are four carbon atoms in the chain containing the carbonyl group, therefore the prefix is butan-. There are no branched chains. This molecule is butanal.
There is a carbonyl group and an oxygen atom attached to two different carbon atoms. This compound must be an ester and have the suffix -oate. There are three carbon atoms in the chain without the carbonyl group and this is propyl. There are two carbon atoms in the chain with the carbonyl group so the prefix is ethan-. There are no branched chains. This molecule is propyl ethanoate.
\(\text{HCOOH}\)
Draw the structural representation:
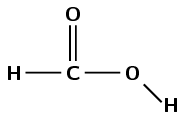
There is a carbonyl group and a hydroxyl group attached to the same carbon atom. This compound must be a carboxylic acid and have the suffix -oic acid. There is one carbon atom in the longest chain therefore the prefix is methan-. There are no branched chains (there can be no branched chains). This molecule is methanoic acid.
Using atomic model kits build molecules of butane, but-1-ene, but-1-yne, butan-1-ol, butanoic acid, butyl butanoate, butan-2-one, butanal. If you don't have an atomic model kit remember that you can use jelly tots (or playdough) and toothpicks. Use different colour jelly tots to represent the different atoms and extra toothpicks to represent double and triple bonds.
Identify the functional group in each of these molecules. Move the functional group around the molecule. You should find that you can do so with but-1-ene, but-1-yne and butan-1-ol. It will not be possible with butanoic acid and there is no functional group to move in butane.
Can you see that if you move the carbonyl group in butan-2-one you will either still get butan-2-one or you will get butanal? Similarly, when moving the carbonyl group in butanal you can only get butanal or butan-2-one.
Build a model of the molecule below:
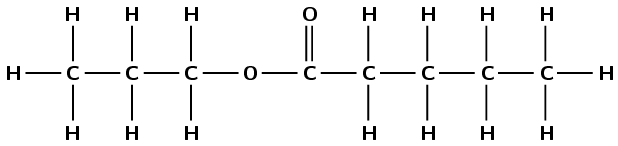
Compare this to butyl butanoate. What differences are there between these two molecules? What similarities are there? What is the name of the new molecule?
Continue by building different compounds for your friends. Make them identify the functional group, the number of carbon atoms and therefore the name of the compound.
Study the table below and answer the questions that follow:
|
A |
B |
C |
|
|
Compound |
Functional group |
Number of carbon atoms |
|
|
1 |
e.g. methane |
e.g. alkane |
e.g 1 |
|
2 |
propanoic acid |
alkyne |
8 |
|
3 |
2-chloroethane |
ketone |
4 |
|
4 |
1-octanal |
carboxylic acid |
6 |
|
5 |
3-heptyne |
aldehyde |
2 |
|
6 |
butanone |
ester |
6 |
|
7 |
3-hexene |
haloalkane |
1 and 5 |
|
8 |
1-hexanol |
alkene |
3 |
|
9 |
methyl pentanoate |
alcohol |
7 |
Match the compounds in column A with the correct functional group in column B. For example methane is an alkane: A1, B1.
A1 (methane), B1 (alkane)
A2 (propanoic acid), B4 (carboxylic acid)
A3 (2-chloroethane), B7 (haloalkane)
A4 (1-octanal), B5 (aldehyde)
A5 (3-heptyne), B2 (alkyne)
A6 (butanone), B3 (ketone)
A7 (3-hexene), B8 (alkene)
A8 (1-hexanol), B9 (alcohol)
A9 (methyl pentanoate), B6 (ester)
Match the compounds in column A with the correct number of carbon atoms in column C. For example methane has one carbon atom in its longest chain: A1, C1.
A1 (methane), C1 (1)
A2 (propanoic acid), C8 (3)
A3 (2-chloroethane), C5 (2)
A4 (1-octanal), C2 (8)
A5 (3-heptyne), C9 (7)
A6 (butanone), C3 (4)
A7 (3-hexene), C4 or C6 (6)
A8 (1-hexanol), C4 or C6 (6)
A9 (methyl pentanoate), C7 (1 and 5)
Match the structural representation in column A with the condensed structural representation (column B) and IUPAC name (column C).
|
A |
B |
C |
|
|
Structure |
Condensed |
IUPAC name |
|
|
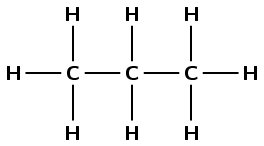
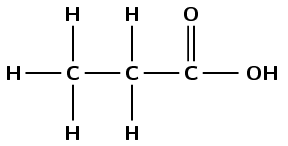
1 |
|
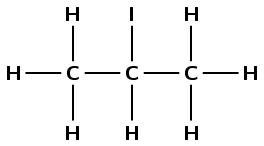
\(\text{CH}_{3}\text{CH}(\text{I})\text{CH}_{3}\) |
ethyl methanoate |
|
2 |
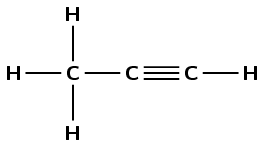
|
\(\text{CH}_{3}\text{CH}_{2}\text{CH}_{3}\) |
propanone |
|
3 |
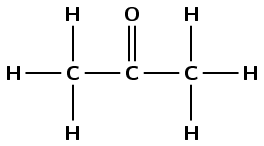
|
\(\text{CH}_{3}\text{COCH}_{3}\) |
propane |
|
4 |
|
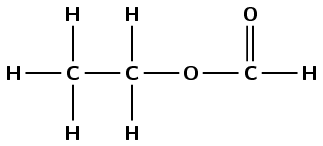
\(\text{HCOOCH}_{2}\text{CH}_{3}\) |
propyne |
|
5 |
|
\(\text{CH}_{3}\text{CCH}\) |
propanal |
|
6 |
|
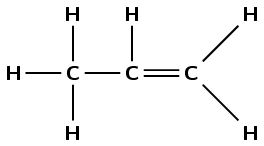
\(\text{CH}_{3}\text{CHCH}_{2}\) |
2-iodopropane |
|
7 |
|
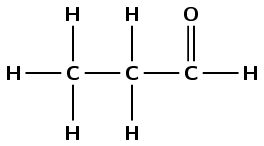
\(\text{CH}_{3}\text{CH}_{2}\text{CHO}\) |
propanoic acid |
|
8 |
|
\(\text{CH}_{3}\text{CH}_{2}\text{COOH}\) |
propene |
propane - A1, B2, C3
propyne - A2, B5, C4
propanone - A3, B3, C2
propanoic acid - A4, B8, C7
2-iodopropane - A5, B1, C6
ethyl methanoate - A6, B4, C1
propene - A7, B6, C8
propanal - A8, B7, C5
Fill in the gaps in the table below:
|
IUPAC name |
Functional group |
Condensed |
Structural |
|
\(\small{{\text{CH}}_{3}{\text{CH}}_{2}{\text{CH}}_{2}{\text{CHO}}}\) |

|
||
|
ethanol |
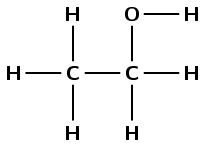
|
||
|
carboxylic acid |
\(\small{{\text{CH}}_{3}{\text{CH}}_{2}{\text{CH(CH}}_{3}{\text{)CH}}_{2}{\text{COOH}}}\) |
||
|
2-methyl pent-2-ene |
\(\small{{\text{CH}}_{3}{\text{C(CH}}_{3}{\text{)CHCH}}_{2}{\text{CH}}_{3}}\) |
||
|
alkane |
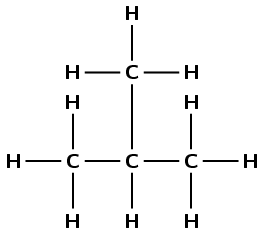
|
||
|
ester |
\(\small{{\text{CH}}_{3}{\text{COOCH}}_{2}{\text{CH}}_{2}{\text{CH}}_{3}}\) |
||
|
butanone |
ketone |
||
|
1-pentyne |
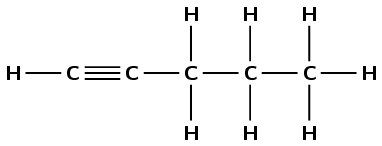
|
||
|
alkyl halide |
\(\small{{\text{CH}}_{2}{\text{(Cl)CH}}_{2}{\text{CH}}_{3}}\) |
|
IUPAC name |
Functional group |
Condensed |
Structural |
|
butanal |
aldehyde |
\(\small{{\text{CH}}_{3}{\text{CH}}_{2}{\text{CH}}_{2}{\text{CHO}}}\) |

|
|
ethanol |
alcohol |
\(\small{{\text{CH}}_{3}{\text{CH}}_{2}{\text{OH}}}\) |

|
|
3-methyl pentanoic acid |
carboxylic acid |
\(\small{{\text{CH}}_{3}{\text{CH}}_{2}{\text{CH(CH}}_{3}{\text{)CH}}_{2}{\text{COOH}}}\) |
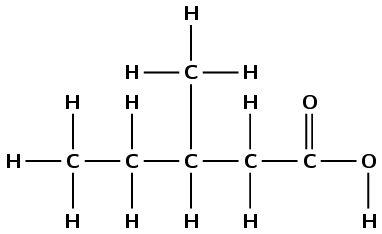
|
|
2-methyl pent-2-ene |
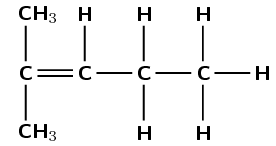
alkene |
\(\small{{\text{CH}}_{3}{\text{C(CH}}_{3}{\text{)CHCH}}_{2}{\text{CH}}_{3}}\) |
|
|
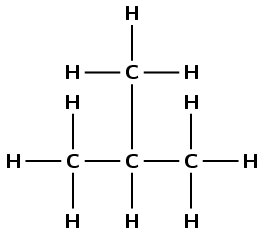
2-methyl propane |
alkane |
\(\small{{\text{CH}}_{3}{\text{CH(CH}}_{3}{\text{)CH}}_{3}}\) |
|
|
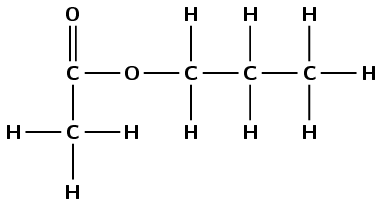
propyl ethanoate |
ester |
\(\small{{\text{CH}}_{3}{\text{COOCH}}_{2}{\text{CH}}_{2}{\text{CH}}_{3}}\) |
|
|
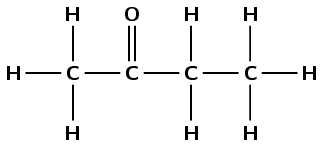
butanone |
ketone |
\(\small{{\text{CH}}_{3}{\text{COCH}}_{2}{\text{CH}}_{3}}\) |
|
|
1-pentyne |
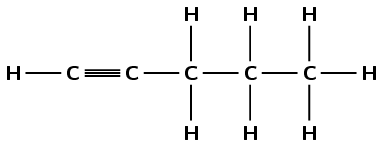
alkyne |
\(\small{{\text{CHCCH}}_{2}{\text{CH}}_{2}{\text{CH}}_{3}}\) |
|
|
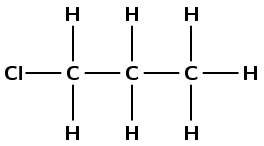
1-chloro propane |
halo alkane |
\(\small{{\text{CH}}_{2}{\text{(Cl)CH}}_{2}{\text{CH}}_{3}}\) |
|
|
Previous
4.2 Organic molecular structures
|
Table of Contents |
Next
4.4 Physical properties and structure
|