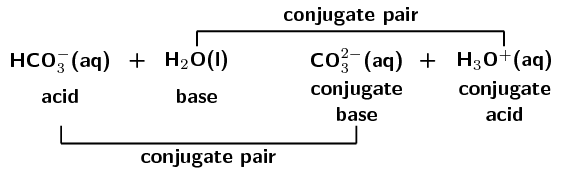
Label the acid-base conjugate pairs in the following equation:
\(\text{HCO}_{3}^{-}(\text{aq}) + \text{H}_{2}\text{O}(\text{ℓ})\)
\(\rightleftharpoons\)
\(\text{CO}_{3}^{2-}(\text{aq}) + \text{H}_{3}\text{O}^{+}(\text{aq})\)
A certain antacid tablet contains \(\text{22,0}\) \(\text{g}\) of baking soda
\((\text{NaHCO}_{3})\). It is used to neutralise the excess hydrochloric acid in the
stomach. The balanced equation for the reaction is:
\(\text{NaHCO}_{3}(\text{s}) + \text{HCl}(\text{aq})\) \(\to\) \(\text{NaCl}(\text{aq}) +
\text{H}_{2}\text{O}(\text{ℓ}) + \text{CO}_{2}(\text{g})\)
The hydrochloric acid in the stomach has a concentration of \(\text{1,0}\)
\(\text{mol·dm$^{-3}$}\). Calculate the volume of the hydrochloric acid that can be
neutralised by the antacid tablet.
(DoE Grade 11 Paper 2, 2007)
\(M(\text{NaHCO}_{3}) = \text{23,0} + \text{1,01} + \text{12,0} + (\text{3}
\times \text{16,0}) = \text{84,01} \text{ g·mol$^{-1}$}\)
\(n\text{(NaHCO}_{3}{\text{)}} = \dfrac{m}{M}\)
\(n = \dfrac{\text{22,0}\text{ g}}{\text{84,01}\text{ g·mol$^{-1}$}}\) =
\(\text{0,26}\) \(\text{mol}\)
From the balanced equation we see that the molar ratio of \(\text{NaHCO}_{3}\) to
\(\text{HCl}\) is \(\text{1}\):\(\text{1}\).
Therefore, \(n(\text{HCl}) = n(\text{NaHCO}_{3}) = \text{0,26} \text{ mol}\)
\(c = \dfrac{n}{V}\) therefore \(V = \dfrac{n}{c}\)
\(V\text{(HCl)} = \dfrac{\text{0,26}\text{ mol}}{\text{1,0}\text{ mol·dm$^{-3}$}}\) =
\(\text{0,26}\) \(\text{dm$^{3}$}\)