Carbon reacts with water according to the following equation:
\[\text{C(s)} + \text{H}_{2}\text{O(g)} → \text{CO(g)} + \text{H}_{2}\text{(g)} \qquad ΔH > 0\]
Is this reaction endothermic or exothermic? Give a reason for your answer.
|
Previous
12.2 Exothermic and endothermic reactions
|
Next
12.4 Chapter summary
|
If you take a match and just hold it or wave it around in the air, the match will not light. You have to strike the match against the side of the box. All chemical reactions need something that makes them start going.
Chemical reactions will not take place until the system has some minimum amount of energy added to it. This energy is called the activation energy.
Activation energy is the minimum amount of energy that is needed to start a chemical reaction.
It is important to realise that even though exothermic reactions release energy they still need a small amount of energy to start the reaction.
Recall from earlier that we drew graphs for the energy changes in exothermic and endothermic reactions. We can now add some information to these graphs. This will also explain why we draw these graphs with a curve rather than using a straight line from the reactants energy to the products energy.
We will start by looking at exothermic reactions. We will use:
\[\text{H}_{2}\text{(g)} + \text{F}_{2}\text{(g)} → 2\text{HF(g)}\]as an example of an exothermic reaction.
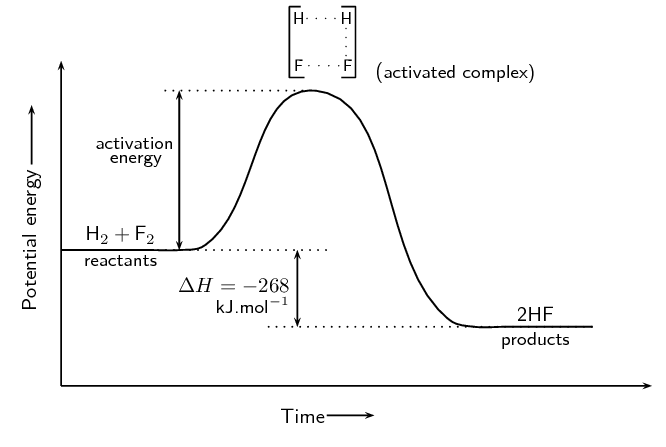
The activation energy is the difference between the energy of the reactants and the maximum energy (i.e. the energy of the activated complex).
The reaction between \(\text{H}_{2} (\text{g})\) and \(\text{F}_{2} (\text{g})\) (Figure 12.4) needs energy in order to proceed, and this is the activation energy. To form the product the bond between \(\text{H}\) and \(\text{H}\) in \(\text{H}_{2}\) must break. The bond between \(\text{F}\) and \(\text{F}\) in \(\text{F}_{2}\) must also break. A new bond between \(\text{H}\) and \(\text{F}\) must also form to make \(\text{HF}\). The reactant bonds break at the same time that the product bonds form.
We can show this as:
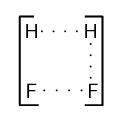
This is called the activated complex or transition state. The activated complex lasts for only a very short time. After this short time one of two things will happen: the original bonds will reform, or the bonds are broken and a new product forms. In this example, the final product is \(\text{HF}\) and it has a lower energy than the reactants. The reaction is exothermic and ΔH is negative.
The reaction between \(\text{H}_{2}\) and \(\text{F}_{2}\) was considered by NASA (National Aeronautics and Space Administration) as a fuel system for rocket boosters because of the energy that is released during this exothermic reaction.
The activated complex is the complex that exists as the bonds in the products are forming and the bonds in the reactants are breaking. This complex exists for a very short period of time and is found when the energy of the system is at its maximum.
Enzymes and activation energy
An enzyme is a catalyst that helps to speed up the rate of a reaction by lowering the activation energy of a reaction. There are many enzymes in the human body, without which lots of important reactions would never take place. Cellular respiration is one example of a reaction that is catalysed by enzymes. You will learn more about catalysts in Grade 12.
In endothermic reactions, the final products have a higher energy than the reactants. An energy diagram is shown below (Figure 12.5) for the endothermic reaction:
\[\text{O}_{2}\text{(g)} + \text{N}_{2}\text{(g)} → 2\text{NO(g)}\]Notice that the activation energy for the endothermic reaction is much greater than for the exothermic reaction.
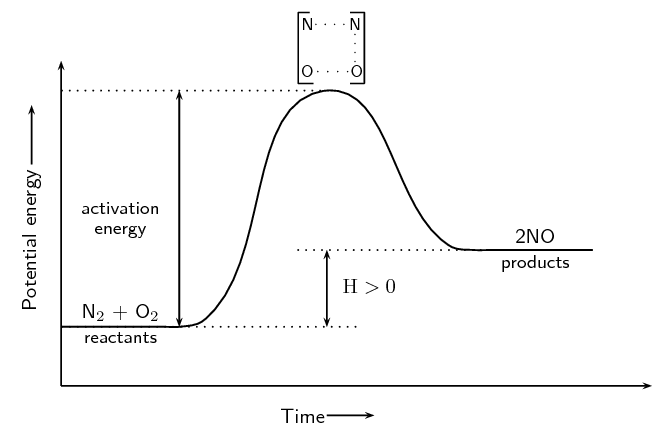
It is because of this activation energy that we first need to show an increase in energy from the reactant to the activated complex and then a decrease in energy from the activated complex to the product. We show this on the energy graphs by drawing a curve from the energy of the reactants to the energy of the products.
Refer to the graph below and then answer the questions that follow:
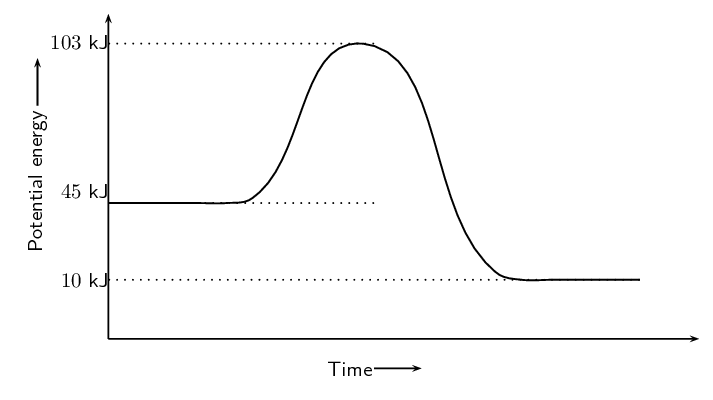
Calculate \(\Delta\)H.
Is the reaction endothermic or exothermic and why?
Calculate the activation energy for this reaction.
\(\Delta\)H is found by subtracting the energy of the reactants from the energy of the products. We find the energy of the reactants and the products from the graph. \begin{align*} \Delta \text{H} & = \text{energy of products} - \text{energy of reactants} \\ & = \text{10}\text{ kJ} - \text{45}\text{ kJ} \\ & = -\text{35}\text{ kJ} \end{align*}
The reaction is exothermic since \(\Delta \text{H} < 0\). We also note that the energy of the reactants is greater than the energy of the products.
The activation energy is found by subtracting the energy of the reactants from the energy of the activated complex. Again we can read the energy of the reactants and activated complex off the graph. \begin{align*} \text{activation energy} & = \text{energy of activated complex} - \text{energy of reactants} \\ & = \text{103}\text{ kJ} - \text{45}\text{ kJ} \\ & = \text{58}\text{ kJ} \end{align*}
Carbon reacts with water according to the following equation:
\[\text{C(s)} + \text{H}_{2}\text{O(g)} → \text{CO(g)} + \text{H}_{2}\text{(g)} \qquad ΔH > 0\]
Is this reaction endothermic or exothermic? Give a reason for your answer.
Refer to the graph below and then answer the questions that follow:
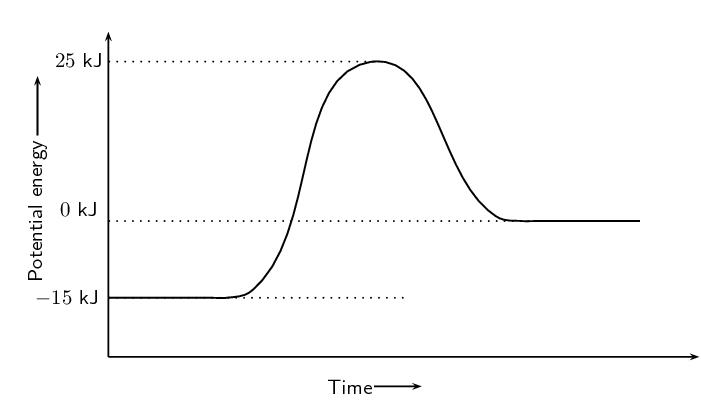
What is the energy of the reactants?
What is the energy of the products?
Calculate ΔH.
We find \(\Delta \text{H}\) using:
\begin{align*} \Delta \text{H} & = \text{energy of products} - \text{energy of reactants} \\ & = \text{0}\text{ kJ} - (-\text{15}\text{ kJ}) \\ & = \text{15}\text{ kJ} \end{align*}What is the activation energy for this reaction?
|
Previous
12.2 Exothermic and endothermic reactions
|
Table of Contents |
Next
12.4 Chapter summary
|