For the following questions state whether they are true or false. If they are
false, correct the statement
-
The group 1 elements are sometimes known as the alkali earth
metals.
-
The group 8 elements are known as the noble gases.
-
Group 7 elements are very unreactive.
-
The transition elements are found between groups 3 and 4.
Solution not yet available.
Give one word or term for each of the following:
-
The energy that is needed to remove one electron from an atom
-
A horizontal row on the periodic table
-
A very reactive group of elements that is missing just one
electron from their outer shells.
Solution not yet available.
Given \(_{35}^{80}\text{Br}\) and \(_{17}^{35}\text{Cl}\). Compare these
elements in terms of the following properties. Explain the differences
in each case.
-
Atomic radius
-
Electronegativity
-
First ionisation energy
-
Boiling point
Solution not yet available.
Given the following table:
Draw graphs to show the patterns in the following physical properties:
-
Density
-
Boiling point
-
Melting point
-
Electronegativity
Solution not yet available.
A graph showing the pattern in first ionisation energy for the elements in
period 3 is shown below:
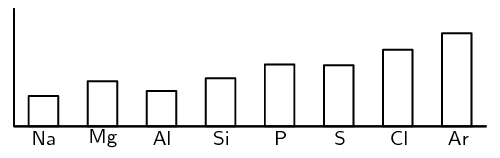
-
Explain the pattern by referring to the electron
configuration
-
Predict the pattern in the first ionisation energy for the
elements in period 2.
-
Draw a rough graph to show the pattern predicted in the
previous question.
Solution not yet available.

