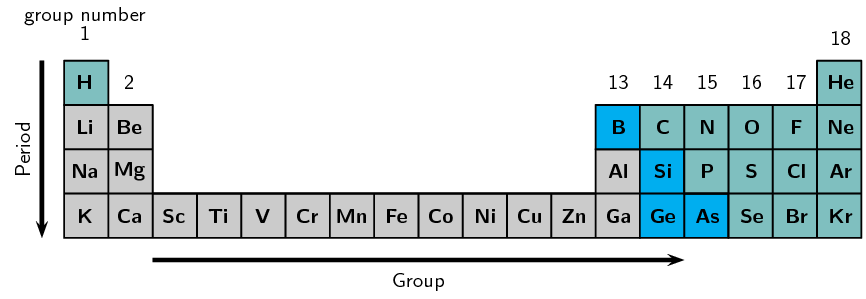
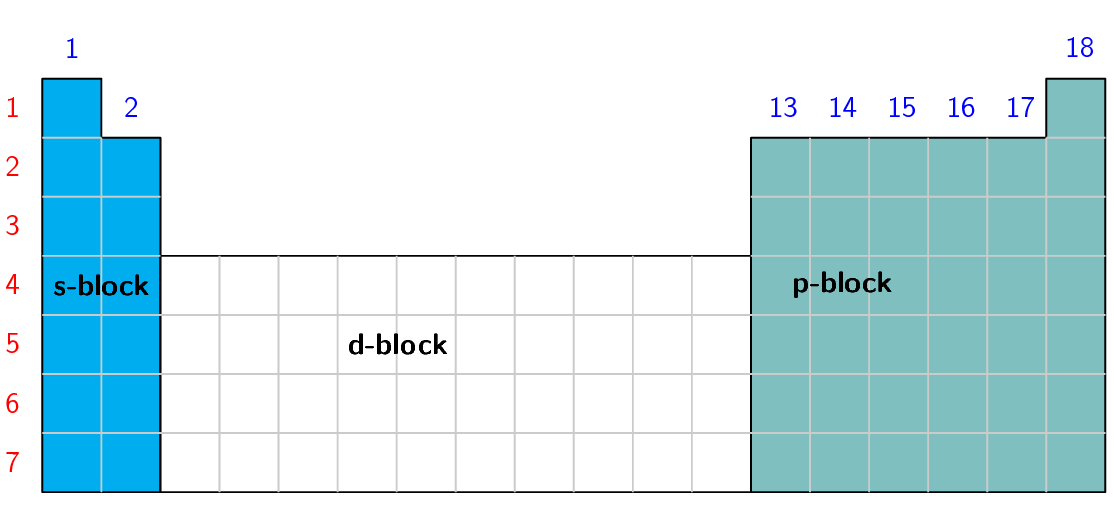
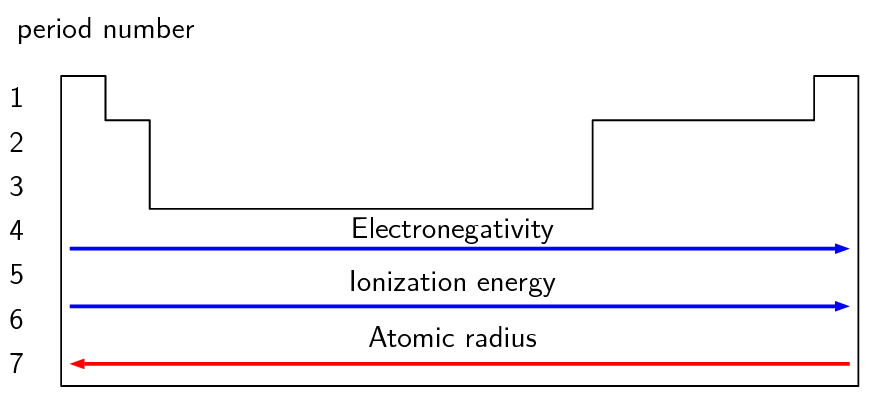
Use Table 5.1 and Figure 5.2 to help you produce a similar table for the elements in period 2.
|
Element |
\(_{3}^{7}\text{Li}\) |
\(_{4}^{9}\text{Be}\) |
\(_{5}^{11}\text{B}\) |
\(_{6}^{12}\text{C}\) |
|
Chlorides |
\(\text{LiCl}\) |
\(\text{BeCl}_{2}\) |
\(\text{BCl}_{3}\) |
\(\text{CCl}_{4}\) |
|
Oxides |
\(\text{Li}_{2}\text{O}\) |
\(\text{BeO}\) |
\(\text{B}_{2}\text{O}_{3}\) |
\(\text{CO}_{2}\) or \(\text{CO}\) |
|
Valence electrons |
\(2\text{s}^{1}\) |
\(2\text{s}^{2}\) |
\(2\text{s}^{2}2\text{p}^{1}\) |
\(2\text{s}^{2}2\text{p}^{2}\) |
|
Atomic radius |
Decreases across the period. |
|||
|
First Ionization energy |
Increases across the period. |
|||
|
Electro-negativity |
Increases across the period. |
|||
|
Melting and boiling point |
Increases to carbon and then decreases to neon. |
|||
|
Electrical conductivity |
Increases to boron and then decreases. Boron is a semi-conductor. Lithium and beryllium are conductors. The rest are insulators. |
|||
|
Element |
\(_{7}^{14}\text{N}\) |
\(_{8}^{16}\text{O}\) |
\(_{9}^{19}\text{F}\) |
\(_{10}^{20}\text{Ne}\) |
|
Chlorides |
\(\text{NCl}_{3}\) |
no compounds, but oxygen does combine with chlorine in compounds called chlorine oxides |
no compounds |
no compounds |
|
Oxides |
\(\text{NO}_{2}\) or \(\text{NO}\) |
No compounds. Oxygen combines with itself to form \(\text{O}_{2}\). |
no oxides, but fluorine does combine with oxygen in compounds called oxygen fluorides. |
no compounds |
|
Valence electrons |
\(2\text{s}^{2}2\text{p}^{3}\) |
\(2\text{s}^{2}2\text{p}^{4}\) |
\(2\text{s}^{2}2\text{p}^{5}\) |
\(2\text{s}^{2}2\text{p}^{6}\) |
|
Atomic radius |
Decreases across the period. |
|||
|
First Ionization energy |
Increases across the period. |
|||
|
Electro-negativity |
Increases across the period. |
|||
|
Melting and boiling point |
Increases to carbon and then decreases to neon. |
|||
|
Electrical conductivity |
Increases to boron and then decreases. Boron is a semi-conductor. Lithium and beryllium are conductors. The rest are insulators. |
|||