Explain the meaning of each of the following terms
- ionic bond
- covalent bond
|
Previous
Chapter summary
|
Next
7.1 Introduction and key concepts
|
Explain the meaning of each of the following terms
Which ONE of the following best describes the bond formed between carbon and hydrogen?
Which of the following reactions will not take place? Explain your answer.
\(\text{H} + \text{H} \rightarrow \text{H}_{2}\)
\(\text{Ne} + \text{Ne} \rightarrow \text{Ne}_{2}\)
\(\text{Cl} + \text{Cl} \rightarrow \text{Cl}_{2}\)
Draw the Lewis structure for each of the following:
calcium
iodine
hydrogen bromide \((\text{HBr})\)
nitrogen dioxide \((\text{NO}_{2})\)
Given the following Lewis structure, where X and Y each represent a different element:
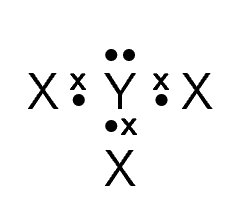
What is the valency of X?
What is the valency of Y?
Which elements could X and Y represent?
Complete the following table:
\(\text{K}^{+}\) | \(\text{Ca}^{2+}\) | \(\text{NH}_{4}^{+}\) | |
\(\text{OH}^{-}\) | |||
\(\text{O}^{2-}\) | |||
\(\text{NO}_{3}^{-}\) | |||
\(\text{PO}_{4}^{3-}\) |
| \(\text{K}^{+}\) | \(\text{Ca}^{2+}\) | \(\text{NH}_{4}^{+}\) | |
| \(\text{OH}^{-}\) | \(\text{KOH}\) | \(\text{Ca}(\text{OH})_{2}\) | \(\text{NH}_{4}\text{OH}\) |
| \(\text{O}^{2-}\) | \(\text{K}_{2}\text{O}\) | \(\text{CaO}\) | \((\text{NH}_{4})_{2}\text{O}\) |
| \(\text{NO}_{3}^{-}\) | \(\text{KNO}_{3}\) | \(\text{Ca}(\text{NO}_{3})_{2}\) | \(\text{NH}_{4}\text{NO}_{3}\) |
| \(\text{PO}_{4}^{3-}\) | \(\text{K}_{3}\text{PO}_{4}\) | \(\text{Ca}_{3}(\text{PO}_{4})_{2}\) | \((\text{NH}_{4})_{3}\text{PO}_{4}\) |
Potassium dichromate is dissolved in water.
Give the name and chemical formula for each of the ions in solution.
What is the chemical formula for potassium dichromate?
Potassium ion: \(\text{K}^{+}\)
Dichromate ion: \(\text{Cr}_{2}\text{O}_{7}^{2-}\)
\(\text{K}_{2}\text{Cr}_{2}\text{O}_{7}\)
|
Previous
Chapter summary
|
Table of Contents |
Next
7.1 Introduction and key concepts
|